Immune checkpoint inhibitors are important therapeutic modalities not only against metastatic melanoma, but in other malignant disorders, including metastatic squamous cell carcinoma, lung, and urogenital carcinomas.1 Albeit the most commonly reported cutaneous toxicities are mild, a subset may persist and can lead to severe or even life-threatening toxicity. Autoimmune bullous disorders belong to the rare adverse events of checkpoint inhibitors, and in a study by Siegel J et al.2 the incidence was found to be approximately 1%. In June 2018, the U.S. Food and Drug Administration (FDA) warned about adverse events of bullous pemphigoid (BP) to be associated with pembrolizumab use. According to a recently published pharmacovigilance analysis of real-world adverse events (including published case reports and reports to the FDA), PD-1 inhibitors, pembrolizumab, and nivolumab were found to have a statically significant signal with BP.3
The authors report two cases of BP which began shortly after the initiation of PD-1 inhibitor therapy. One of the patients presented with metastatic melanoma and received nivolumab therapy (case 1), while the other one was treated with pembrolizumab for his urothelial carcinoma (case 2). In both cases, the diagnosis of BP was based on clinical symptoms and confirmed by histopathological and direct immunofluorescence findings. At the time of diagnosis, elevated BP antibody titers were only present in case 2 (elevated serum levels of BP180). In case 1, during the nivolumab treatment palliative radiotherapy was initiated for the metastatic lesions of the right leg with mild symptoms of BP present at that time. In both cases, methylprednisolone (0.5–1.0 mg/kg) was eventually administered and resulted in complete healing of the skin lesions (Figure 1). In case 1, BP flare was seen after steroid taper; therefore, her regular oral antidiabetic drug (a gliptin derivate) was switched to metformin and an oral corticosteroid was readministered. After cessation of oral steroids, a challenge with nivolumab was initiated. After the second cycle of nivolumab vesicles appeared again, and increased levels of BP230 were detected by ELISA. Symptoms resolved with topical clobetasol propionate therapy, and after complete regression pembrolizumab was started. Until now, the patient has received a total of nine cycles of pembrolizumab, and no blisters have occurred, while her melanoma is in stable condition. In case 2, BP reoccurred despite the slow tapering of the corticosteroid therapy. Methylprednisolone was reintroduced, but unfortunately his tumour progressed and the patient deceased in September 2018.
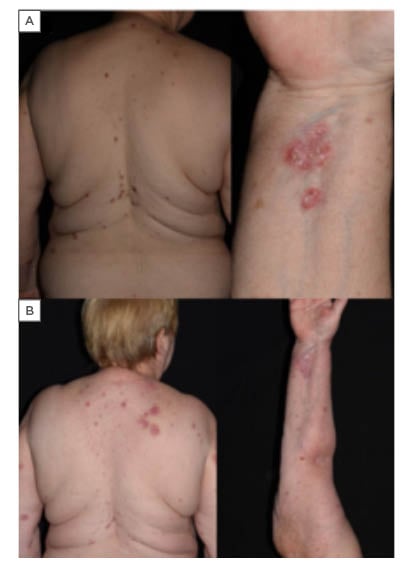
Figure 1: A) Clinical images of bullous pemphigoid (case 1). Extensive pruritic skin lesions, erythematous plaques with erosions. B) Healing of the lesions on methylprednisolone therapy.
In summary, in both presented cases skin symptoms occurred early during the course of PD-1 inhibitor treatment (9 weeks and 12 weeks), which is in line with current literature data. Systemic steroid therapy resulted in complete clearance of the skin lesions; however, even after slow tapering and discontinuation of PD-1 inhibitor therapy, flares of BP were seen. In case 1, concurrent radiation therapy might have aggravated the course of BP, and the regularly taken gliptin derivate may have potentiated the risk of BP. Interestingly, ELISA was negative in case 1 and upon challenging with nivolumab she became positive for BP230, which has been reported in PD-1 inhibitor induced BP less commonly. The results suggest the possibility of an epitope spreading phenomena. Overall, patients on checkpoint inhibitors should be carefully monitored for any subtle skin symptoms of BP, and the risks and benefits of PD-1 inhibitors weighted when deciding on whether to continue the medication.







