Abstract
Psoriasis is a chronic, immune-mediated skin condition with systemic involvement, frequently requiring long-term treatment. At present, there are 11 biologic agents available for the treatment of moderate-to-severe psoriasis, which target specific inflammatory cytokines involved in the immunopathogenesis of the disease. Among these, three monoclonal antibodies specifically inhibit the p19 subunit of IL-23. IL-23 is a heterodimeric cytokine consisting of two subunits: IL-23p19 and IL-23p40. IL-23 plays a key role in the immunopathogenesis of psoriasis by activating Th17 cells, leading to stimulation of downstream cytokines involved in the systemic inflammation and keratinocyte hyperproliferation observed in psoriasis. Overall, the anti-IL-23 agents demonstrate rapid clinical improvement along with a favourable safety profile. This review has analysed data on the clinical efficacy, safety, and tolerability of the three IL-23 agents (tildrakizumab, guselkumab, and risankizumab) in the treatment of moderate-to-severe plaque psoriasis.
INTRODUCTION
Psoriasis is a common, chronic, immune-mediated skin disease affecting approximately 2–3% of the global population.1,2 Characteristic signs and symptoms of psoriasis include well-demarcated erythematous plaques with silvery scales, and significant pruritus and discomfort, which often impacts psychosocial function and reduces quality of life amongst patients.
Standardised scoring systems used to evaluate these signs and symptoms of psoriasis can be found in Table 1. Comorbid conditions linked to psoriasis include cardiovascular disease, metabolic syndrome, psychosocial disorders, and psoriatic arthritis.6-8
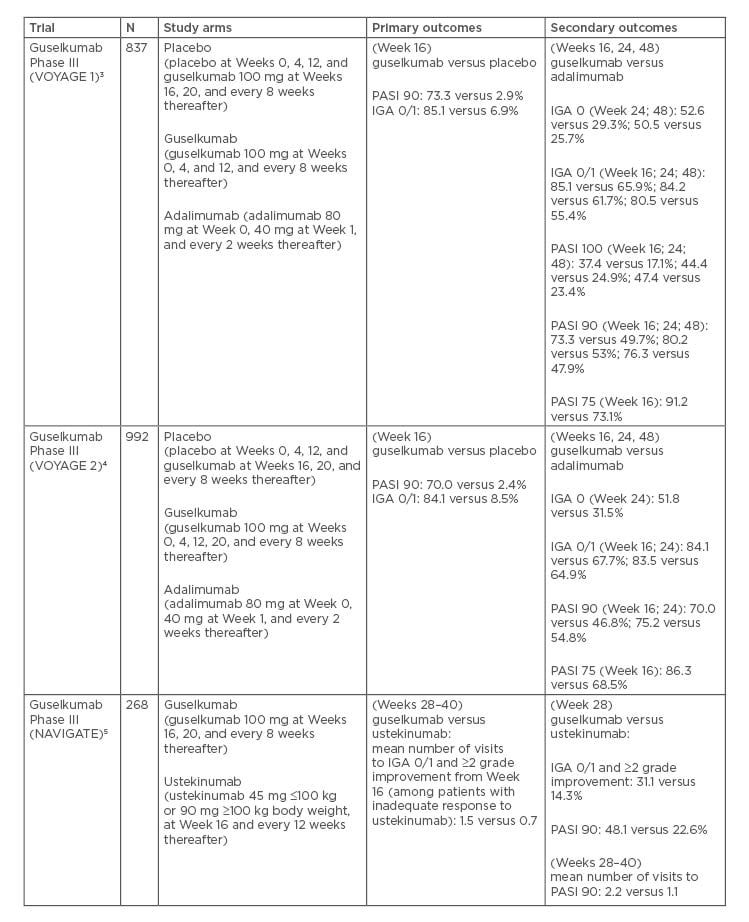
Table 1: Pivotal Phase III trials for guselkumab.
The role of T lymphocytes in psoriatic disease has long been acknowledged. More recently, Th17 cells and the associated IL-23/IL-17 pathway have emerged as central to the immunopathogenesis of psoriasis. IL-23 is a heterodimeric cytokine consisting of two subunits: IL-23p19 and IL-23p40. IL-23 is produced by dendritic cells and keratinocytes, among others, causing the proliferation and survival of Th17 cells, as well as the production of IL-17A and IL-22, which are key drivers of the keratinocyte proliferation central to psoriasis.9,10 Clinical trials for psoriasis treatments have successfully used monoclonal antibodies against IL-17 and IL-23, supporting the current evidence of these cytokines as key drivers of psoriasis.
Three IL-17 antagonists, secukinumab, ixekizumab, and brodalumab have been approved for the treatment of psoriasis, with additional IL-17 inhibitors under development. In addition, ustekinumab is a combined IL-12 and IL-23 blocker approved for the treatment of moderate-to-severe plaque psoriasis (MTSPP), psoriatic arthritis, and Crohn’s disease. Ustekinumab binds to the p40 subunit common to IL-12 and IL-23, preventing its interaction with the IL-12 receptor β1 subunit present on IL-12 and IL-23 receptor complexes.11
Most recently, increasingly specific therapeutic agents targeted to the p19 subunit of IL-23 to inhibit the release of proinflammatory cytokines have been developed and approved. As a result of their specificity, favourable safety profiles and efficacies have been observed. Three p19 IL-23 inhibitors are approved by the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA). This report focusses specifically on these inhibitors, summarising the results of Phase III clinical trials for guselkumab, tildrakizumab, and risankizumab (Table 2). Their clinical efficacy, safety, and tolerability in the treatment of MTSPP will be reviewed.
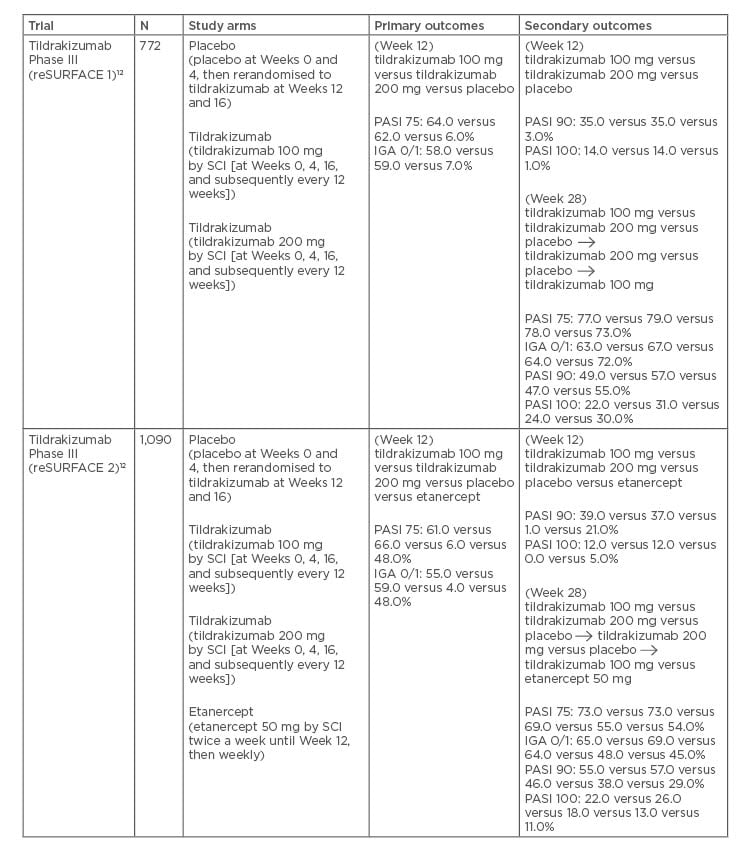
Table 2: Pivotal Phase III trials for tildrakizumab.
GUSELKUMAB
Guselkumab is a fully human immunoglobulin G1 (IgG1)λ monoclonal antibody that selectively binds to the p19 subunit of IL-23. Guselkumab decreases levels of IL-17A in both the serum and skin lesions of psoriasis patients.13 By targeting the p19 subunit, guselkumab is able to specifically inhibit IL-23, in contrast to ustekinumab which targets both IL-12 and IL-23 via binding to the shared p40 subunit. As a result, guselkumab leaves the IL-12/Th1 axis undisturbed, leaving an important regulator of immune function intact.14 Guselkumab was first approved by the FDA in July 2017 and the EMA in November 2017, making it the first in the IL-23 class to be approved in adults with MTSPP in the USA and Europe.
Dosage
The recommended dosage of guselkumab for adult patients with MTSPP is 100 mg by subcutaneous injection at Weeks 0 and 4, and every 8 weeks thereafter.
Clinical Efficacy
Several clinical trials have assessed the clinical efficacy and safety of guselkumab. VOYAGE 1 and VOYAGE 2 were randomised, double-blind, pivotal Phase III clinical trials performed with guselkumab, placebo, and a comparator, adalimumab (a TNF-α inhibitor) for the treatment of MTSPP.3,4 Both were 48-week studies, comparing guselkumab to placebo and adalimumab. Patients were randomised at baseline to receive either a placebo at Weeks 0, 4, and 12, followed by guselkumab 100 mg at Weeks 1 and 20, and then every 8 weeks; guselkumab 100 mg at Weeks 0, 4, and every 8 weeks thereafter; or adalimumab 80 mg at Week 0, adalimumab 40 mg at Week 1 and adalimumab 40 mg every 2 weeks thereafter.15 Additionally, VOYAGE 2 investigated the maintenance of efficacy of guselkumab after withdrawal.
Guselkumab was significantly superior to placebo and to adalimumab in both VOYAGE trials at 16 weeks for its 2 primary outcomes: the Investigator’s Global Assessment (IGA) scale of 0 or 1 (VOYAGE 1/2: 85.1/84.1% guselkumab versus 6.9/8.5% placebo) and the Psoriasis Area and Severity Index (PASI) 90 response (VOYAGE 1/2: 73.3/70.0% guselkumab versus 2.9/2.4% placebo).3,4
Compared to adalimumab, guselkumab was also found to be significantly superior as measured by the proportion of patients achieving IGA 0 or 1 (VOYAGE 1/2: 85.1/84.1% guselkumab versus 65.9/67.7% adalimumab) and PASI 90 (73.3/70.0% guselkumab versus 47.9/46.8% adalimumab) at Week 16. Significantly better responses to guselkumab compared with adalimumab were also maintained at Week 24. At Week 48 of VOYAGE 1, the rates comparing guselkumab against adalimumab for IGA 0, IGA 0/1, and PASI 90 were 50.5% versus 25.7%, 80.5% versus 55.4%, and 76.3% versus 47.9%, respectively.3
Additionally, VOYAGE 2 investigated the efficacy of guselkumab after withdrawal.4 Patients with ≥90% PASI improvement from baseline were re-randomised to a withdrawal group at Week 28. Patients received either placebo or maintenance therapy at this point.
The guselkumab withdrawal group restarted Guselkumab either upon loss of ≥50% of Week 28 PASI improvement or by Week 72. VOYAGE 2 reported superior maintenance of response from Weeks 28 to 48 in patients maintained on guselkumab compared to those who underwent withdrawal (88.6% versus 36.8%; p<0.001).
Additionally, in the adalimumab nonresponders who were switched to guselkumab, 66.1% achieved PASI 90 at Week 48, with 28.6% achieving PASI 100.4 When compared to the maintenance group sustained through Week 72, the efficacy in the guselkumab withdrawal group had diminished (11.5% versus 86.0%).
After 20 weeks of retreatment, 80.4% of guselkumab withdrawal patients achieved PASI 90 responses compared to baseline.16 Furthermore, PASI improvements correlated with improvement in anxiety (r=0.27; p<0.0001) and depression (r=0.25; p<0.0001) scores in patients with baseline Hospital Anxiety and Depression Scale (HADS) of ≥8. Greater improvements in HADS were also observed at Week 16 in guselkumab-treated versus placebo-treated patients using a stricter cut-off of 11 on the HADS.17 In addition, considerably greater improvements from baseline were observed at Weeks 8 and 16 in the guselkumab group compared to placebo when using the Dermatology Life Quality Index (DLQI). Guselkumab showed significantly greater improvement over adalimumab at Week 24 using the DLQI (p<0.001). The proportion of patients achieving DLQI 0 or 1 (indicating no impact) at Week 24 was higher with guselkumab compared to adalimumab (58.9 versus 40.2%; p<0.001).18
Pooled analysis of both VOYAGE trials evaluating the effect of guselkumab on psoriasis in specific body regions revealed that guselkumab was superior to placebo in the treatment of scalp psoriasis, traditionally a region ‘difficult to treat’.19 The proportion of patients achieving a scalp specific-IGA score of 0 or 1 was 81.8% for guselkumab versus 12.4% for placebo at Week 16. When compared to adalimumab (68.5%), guselkumab was also superior (85.0%) at Week 24 (p<0.001). Furthermore, a greater percentage of the guselkumab group versus the adalimumab group achieved a scalp specific-IGA score of 0 (69.9% versus 56.3%; p<0.001). Palmoplantar psoriasis, another difficult-to-treat area, was also evaluated. The Physician’s Global Assessment of the Hands and Feet (hf-PGA) score of 0 or 1 was achieved by 75.5% in the guselkumab group versus 14.2% in the placebo group at Week 16, and 80.4% in the guselkumab group versus 60.3% in the adalimumab group at Week 24. A greater percentage of the guselkumab group versus the adalimumab group achieved a hf-PGA score of 0 (75.0% versus 50.3%; p<0.001). The difference in finger-PGA score of 0 or 1 was also statistically significant between guselkumab and placebo at Week 16 (46.7% versus 15.2%; p<0.001), but was not when compared to adalimumab at Week 24 (60.0% versus 64.3%; p=0.11).
Three-year long-term efficacy data for continuous treatment with guselkumab has been reported from the Phase III VOYAGE 1 trial.20 Clinical responses for guselkumab were maintained through Week 156 in the open-label extension. The proportions of patients who achieved PASI 75, PASI 90, PASI 100, IGA 0/1, and IGA 0 at Week 156 were 96.0%, 82.8%, 50.8%, 82.1%, and 53.1%, respectively. Psoriasis Symptoms and Signs Diary (PSSD) responses were maintained at Week 100 and Week 156 with 40.2% and 40.4% of patients reporting a PSSD score of 0 in both instances, respectively.21
A Japanese study with 192 patients reported similar results.5 At Week 16, a significantly higher proportion of patients receiving guselkumab 50 mg or 100 mg versus placebo achieved IGA 0 or 1 (92.3%, 88.9%, and 7.8%, respectively) and PASI 90 (70.8%, 69.8%, and 0.0%, respectively). Patients in the guselkumab 50 mg and 100 mg groups achieved significant improvements in PASI 75 compared to placebo at Week 16 (89.2%, 84.1%, and 6.3%, respectively). Improvements were maintained through Week 52.
NAVIGATE was a Phase III randomised, double-blind trial that evaluated the efficacy and safety of guselkumab in 268 patients with MTSPP who had a previous inadequate response to ustekinumab.22 All enrolled patients initially received ustekinumab 45 mg or 90 mg (weight-based dosing) at Weeks 0 and 4. Patients were then assessed for IGA response at Week 16. Those with a continued IGA score ≥2 at Week 16 were randomised either to continue ustekinumab (every 12 weeks) or to switch to guselkumab 100 mg (at Weeks 16, 20, and then every 8 weeks thereafter). Those with an IGA ≤1 continued to receive ustekinumab every 12 weeks. The primary endpoint, the mean number of visits at which patients achieved IGA of 0 or 1 and at least a 2-Grade improvement, was significantly greater in the guselkumab group compared to those who were maintained on ustekinumab (1.5 versus 0.7; p<0.001). Greater proportions of patients in the guselkumab group achieved IGA 0 or 1 and at least a 2-Grade improvement at Week 28 compared to ustekinumab (31.1% versus 14.3%; p=0.001) and Week 52 (36.3% versus 17.3%; p<0.001).21 At Week 52, a significantly greater proportion of patients who transitioned to guselkumab (51.1%) achieved PASI 90 compared to those who continued on ustekinumab (24.1%).
A head-to-head comparison between guselkumab and secukinumab (ECLIPSE) randomised 1,048 psoriasis patients to 1 of 2 groups: 100 mg guselkumab at Weeks 0, 4, and every 8 weeks thereafter; or 300 mg secukinumab weekly for 5 weeks and every 4 weeks thereafter.23 The primary endpoint of PASI 90 response showed guselkumab patient response to be superior at Week 48 (84.5% guselkumab versus 70.0% secukinumab; p<0.001). Secondary endpoints were PASI 75 and IGA 0 responses at Weeks 12 and 48. In guselkumab patients, 84.6% achieved PASI 75 at Week 48 compared to 80.2% of those taking secukinumab, showing noninferiority (p<0.001) but not superiority (p=0.062). IGA 0 at Week 48 was achieved by 62.2% of patients on guselkumab and 50.4% on secukinumab. Complete clearance (PASI 100) was reported in 58.2% of guselkumab patients compared to 48.4% of those on secukinumab at Week 48.
A study evaluating the utility of guselkumab in the treatment of generalised pustular psoriasis and erythrodermic psoriasis revealed a 77.8% and 90.9% treatment success rate in both sets of patients at Week 16, respectively.24 Furthermore, treatment with guselkumab consistently showed improvement in response for secondary endpoints such as PASI, IGA, Japanese Dermatological Association (JDA) severity index, and improvement in body surface area.
Case reports suggest that guselkumab therapy could be effective for paradoxical psoriatic alopecia induced by adalimumab or brodalumab.25 It has also shown effectiveness in patients with concomitant Crohn’s disease who achieve remission while undergoing treatment for psoriasis.25,26 Future therapeutic indications of guselkumab include palmoplantar pustulosis, psoriatic arthritis, and hidradenitis suppurativa, with early studies showing promising treatment responses.27-29
Adverse Reactions
The most common adverse events (AE) reported in the VOYAGE trials included nasopharyngitis, upper respiratory tract infection, erythema at the injection site, and headache.3 Serious infections requiring antibiotic treatment occurred in similar rates across both the guselkumab and adalimumab groups. Serious AE, including those that led to study agent discontinuation, occurred infrequently and in similar proportions of patients for each treatment group. Incidence rates of candidiasis and neutropaenia were low and also comparable between groups.3 In the NAVIGATE trial, infection was the most common AE.22 Of the patients given guselkumab, 77.9% reported at least 1 AE compared to 81.6% of those administered secukinumab. In the ECLIPSE trial, serious AE were reported in 6.2% of guselkumab patients and 7.2% of secukinumab patients. At 44 weeks, 5.1% of the patients on guselkumab had discontinued therapy compared to 9.3% on secukinumab.23 There has also been a reported case of nummular dermatitis associated with guselkumab treatment for palmoplantar psoriasis, as well as a report of a patient developing multiple lentigines following treatment of psoriasis with guselkumab.30,31
TILDRAKIZUMAB
Tildrakizumab is a high-affinity, humanised, IgG1κ monoclonal antibody that targets the p19 subunit of IL-23. It received FDA approval in March 2018 and EMA approval in September 2018 for the treatment of MTSPP.
Dosage
The recommended dose for tildrakizumab for adult patients with MTSPP is a 100 mg, subcutaneous injection at Weeks 0, 4, and every 12 weeks thereafter.
Clinical Efficacy
Several studies have evaluated the efficacy and safety of tildrakizumab. Both of the pivotal Phase III reSURFACE trials were three-group, parallel, double-blind, randomised, placebo-controlled studies.12 In reSURFACE 1, 772 patients with moderate-to-severe chronic plaque psoriasis were randomised to receive tildrakizumab 100 mg, tildrakizumab 200 mg, or placebo (2:2:1) at Weeks 0, 4, and 16. At Week 12, the placebo patients crossed over to receive tildrakizumab at 100 mg or 200 mg for Weeks 12 and 16. At Week 12, the proportion of patients on either dose of tildrakizumab achieving PASI 75 compared to placebo was significantly greater (64.0% on 100 mg, 62.0% on 200 mg, and 6.0% on placebo). When comparing PGA responses in the tildrakizumab groups compared to placebo, 58.0% in the 100 mg group, 59.0% in the 200 mg group, and 7.0% in the placebo group achieved a PGA score of 0 or 1, with ≥2 Grade reductions from baseline at 12 weeks (p<0.0001). Long-term extension data from reSURFACE 1 at Week 160 showed that patients on tildrakizumab 100 mg and 200 mg achieved high and durable PASI 75/90/100 response rates of 84.4%, 57.6%, and 24.9%, and 75.4%, 50.8%, and 25.4%, respectively.
In reSURFACE 2, 1,090 patients were divided into 4 treatment groups: tildrakizumab 200 mg; tildrakizumab 100 mg at Weeks 0, 4, and 16; placebo; or etanercept 50 mg twice weekly until Week 12, then once a week until Week 28 (2:2:1:2).32 At Week 12 of reSURFACE 2, 66.0% in the 200 mg group, and 61.0% in the 100 mg group achieved PASI 75, compared to 6.0% in the placebo group and 48.0% in the etanercept group. A total of 59.0% in the 200 mg and 55.0% in the 100 mg tildrakizumab groups achieved a significant PGA response, compared to 4.0% in the placebo group and 48.0% of patients receiving etanercept.32 At Week 148 in the reSURFACE 2 extension study, tildrakizumab again demonstrated sustained clinical response with PASI 75, 90, and 100 achieved by 89.0%, 64.0%, and 35.0% of patients, respectively. 33
Pooled analysis from 3 clinical trials revealed that at Week 12, PASI and PGA responses to tildrakizumab versus placebo were numerically greater in patients with lower versus higher bodyweight, and that responses were better on 200 mg compared to 100 mg of tildrakizumab for those with higher bodyweight.34 At Week 12, the proportion of patients achieving PASI 75 on tildrakizumab 100 mg or 200 mg was higher compared to placebo in patients both with and without prior exposure to biologic therapy for psoriasis. The proportions of patients with PASI 90 and PGA responses on both tildrakizumab 100 mg and 200 mg versus placebo were greater in biologic naïve patients compared to those with prior biologic exposure.
Pooled analysis from the reSURFACE 1 and 2 trials investigating long-term outcomes found that at Week 148, the tildrakizumab 200 mg responder group (≥75% improvement in PASI) and partial responder group (≥50 to <75% improvement in PASI) had a higher percentage of responders achieving a PASI of 75, 90, and 100, compared to the tildrakizumab 100 mg group.35
Adverse Reactions
The most common adverse reaction reported in both Phase III reSURFACE trials up to Week 28 was nasopharyngitis. The incidence of severe infection, malignancies (including non-melanoma skin cancer), and major cardiovascular AE were low and similar across all treatment groups.32 Furthermore, analysis of the adverse events reported from Phase IIb and two Phase III (reSURFACE 1 and reSURFACE 2) trials suggested that the IL-23 inhibitor tildrakizumab does not induce or worsen inflammatory bowel disease (IBD) in patients with psoriasis, in contrast to the IL-17 class of inhibitors.36-39
Safety and tolerability up to 64 weeks of tildrakizumab therapy using pooled data from 3 randomised controlled trials showed that in the full trial period, exposure-adjusted rates (patients per 100 patient-years), treatment-emergent serious AE, and discontinuations due to AE with tildrakizumab 100 mg and 200 mg, were lower than or comparable with the placebo rates, and lower than with etanercept.40 Pooled analysis from the reSURFACE 1 and 2 trials for the rates of discontinuation of tildrakizumab due to AE, major cardiovascular AE, severe infection, and malignancy were low, and tildrakizumab efficacy was well maintained in Week 28 responders who continued tildrakizumab treatment for 3 years.35
RISANKIZUMAB
Risankizumab is a humanised IgG1 monoclonal antibody that binds to the p19 subunit of IL-23, working similarly to both guselkumab and tildrakizumab. It received FDA and EMA approval in April 2019 for the treatment of MTSPP, making it the most recently approved IL-23p19 inhibitor. Risankizumab is currently being investigated for its utility in Crohn’s disease, whereas other studies have revealed its lack of efficacy in the treatment of ankylosing spondylitis (AS).41-43
Dosage
The recommended dosage for risankizumab for the treatment of MTSPP is 150 mg administered by subcutaneous injection at Weeks 0, 4, and every 12 weeks thereafter.
Clinical Efficacy
In a Phase II clinical trial, 77.0% of patients treated with risankizumab achieved a PASI 90 response at Week 12, compared to 40.0% of patients on ustekinumab (p<0.001).44 Furthermore, risankizumab has been evaluated in two pivotal, identical, Phase III, double-blind, randomised controlled trials (UltIMMa-1 and UltIMMa-2).45,46 Patients were randomised to 1 of 3 groups: risankizumab (150 mg), ustekinumab (45 mg or 90 mg depending on weight), or placebo at Weeks 0 and 4. At Week 16, patients in the placebo group were switched to risankizumab, administered at Weeks 16, 28, and 40. In UltIMMa-1, PASI 90 was achieved by 75.3% of patients receiving risankizumab by Week 16, compared to 42.0% receiving ustekinumab and 4.9% receiving placebo (p<0.001). Static PGA of 0 or 1 was achieved by 87.6% of patients receiving risankizumab by Week 16, compared to 63.0% receiving ustekinumab and 7.8% receiving placebo (p<0.001).45 In UltIMMa-2, 83.7% of patients receiving risankizumab versus 5.1% receiving placebo (placebo-adjusted difference 78.5% [95% CI; 72.4–84.5]) and 61.6% receiving ustekinumab achieved static PGA 0 or 1 at Week 16 (ustekinumab-adjusted difference 22.3% [12.0–32.5]; p<0.0001).46 In UltIMMa-1 and UltIMMa-2, 81.9% and 80.6% of patients treated with risankizumab achieved PASI 90 at 52 weeks, compared to 44.0% and 50.5% in the ustekinumab groups, and 78.4% and 85.1% in the crossover groups, respectively. PASI 100 was achieved at Week 16 by 36.0% and 51.0% of patients treated with risankizumab in UltIMMa-1 and 2, respectively, compared to 12.0% and 24.0% of ustekinumab patients. At Week 52, 56.0 % and 60.0% of patients treated with risankizumab achieved PASI 100 in UltIMMa-1 and 2, respectively, compared to 21.0% and 30.0% of patients treated with ustekinumab.46
A Japanese Phase II/III, double-blinded, placebo-controlled study (SustaIMM) stratified 171 patients with MTSPP by bodyweight and concomitant psoriatic arthritis.47 Patients were randomised to risankizumab 75 mg, 150 mg, or placebo at Weeks 0, 4, 16, 28, and 40 (with placebo crossover to 150 mg occurring at Week 16). Primary endpoints were PASI 90 at Week 16 for risankizumab 75 mg, 150 mg, and placebo, with significantly higher response rates for patients receiving risankizumab 75 mg and 150 mg compared to placebo (75.9% versus 74.5% versus 1.7%, respectively; p<0.001). Week 16 PASI 90 response was seen in 72.7%, 100.0%, and 0.0% of patients with psoriatic arthritis receiving risankizumab 75 mg, risankizumab 150 mg, and placebo, respectively. Patients weighing ≤90 kg (without psoriatic arthritis) and those >90 kg were also compared at the same dosages, with PASI 90 response seen in 80.0%, 69.8%, and 2.3%, and 57.1%, 85.7%, and 0.0% of the patients, respectively. Secondary endpoints included PASI 75, PASI 100, and IGA 0/1 responses which were significantly higher for both risankizumab doses (75 mg and 150 mg) compared to placebo (PASI 75: 90.0%, 95.0%, 9.0%; PASI 100: 22.0%, 33.0%, 0.0%; IGA 0/1: 86.0%, 93.0%, 10.0%, respectively). PASI and IGA response rates were maintained or improved through Week 52. The American College of Rheumatology (ACR)20 responses were measured in 11 patients at select study sites, with ACR20 higher in those on risankizumab versus placebo at Week 16. Sustained efficacy was confirmed in 55% of patients at Week 52.
More recently, risankizumab was compared with adalimumab in patients with MTSPP in an active comparator-controlled, Phase III trial.
IMMvent randomised patients 1:1 to risankizumab 150 mg at Weeks 0 and 4, or to adalimumab 80 mg at Week 0 and then adalimumab 40 mg at Weeks 1, 3, 5, and every other week thereafter until Week 16 (the end of the double-blind treatment period).48 During Weeks 16–44, adalimumab intermediate responders either continued on adalimumab or switched to risankizumab (1:1). Randomisation was stratified by weight and prior TNF-inhibitor exposure. The primary endpoints were PASI 90 and IGA 0/1 at Week 16 and PASI 90 in intermediate responders at Week 44. Risankizumab showed significantly greater efficacy than adalimumab for all primary endpoints: PASI 90: 72.0% risankizumab versus 47.0% adalimumab and IGA 0/1: 84.0% risankizumab versus 60.0% adalimumab.
At Week 44, PASI 90 response among adalimumab intermediate responders was reached by 66.0% of those switched to risankizumab versus 21.0% of those who continued adalimumab.
IMMhance was another Phase III, randomised, double-blind, placebo-controlled study including randomised withdrawal and retreatment. Initially, 507 patients were assigned to receive either 150 mg risankizumab or placebo (4:1).
Of the patients receiving risankizumab, 73.2% achieved PASI 90 at Week 16, compared to 2.0% of patients receiving placebo (p<0.001). Furthermore, 83.5% of patients on risankizumab achieved sPGA scores of 0 or 1, compared to 7.0% of patients on placebo. Patients on placebo were switched to active drug at Week 16. At Week 28, patients who had achieved sPGA of 0 or 1 were either re-randomised to continue on risankizumab or switch to placebo. At Week 52, 52.4% of those re-randomised to the placebo group achieved PASI 90 compared to 85.6% of those who continued on risankizumab, with sPGA 0/1 scores achieved by 61.3% and 87.4% of patients, respectively.49
Adverse Reactions
In the Phase II trials with risankizumab, rates of serious AE in the 18 mg and 90 mg risankizumab groups, and the ustekinumab group were 12.0%, 15.0%, and 8.0%, respectively. Serious AE included 2 basal cell carcinomas and 1 major cardiovascular AE; there were no serious AE in the 180 mg risankizumab group.44 In the Phase III UltLMMa trials, the frequencies of AE in the risankizumab, ustekinumab, and placebo groups were similar during the first 16 weeks. The most commonly reported AE were upper respiratory tract infection, headache, fatigue, injection site reactions, and tinea infections.45,50 No additional, unexpected safety concerns for risankizumab emerged during the SustaIMM or IMMvent trials (including for those patients who switched from adalimumab to risankizumab) compared to previous Phase III trials. AE in these trials were also comparable to those of the UltLMMa trials.47,48
CONCLUSION
This review of the IL-23 inhibitors (guselkumab, tildrakizumab, and risankizumab) illustrates the significant clinical efficacy and safety of these agents for MTSPP. The development and approval of these agents has expanded psoriasis treatment with increasingly effective biologic options,51 validating the importance of IL-23 as a key cytokine in psoriasis pathogenesis and establishing PASI 90 and 100 as new specific endpoints in clinical trials, compared to the prior PASI 75 standard. Clinical trials with IL-23 agents have also yielded favourable safety data in the treatment of MTSPP.
In this review, the main results from Phase III clinical trials have been summarised. For all three agents, the most common adverse effects reported in the IL-23 clinical trials were similar to other psoriasis biologics, i.e., nasopharyngitis and upper respiratory infection, with rates of serious AE comparable to placebo. Selectively targeting the p19 subunit of IL-23 is of importance to avoid side effects seen with other classes of biologics (for example, risk of tuberculosis reactivation with TNF-α inhibitors, fungal infections with IL-17 inhibitors, etc.). Although data from clinical trials are extremely promising showing impressive clinical efficacy and no new safety signals to date, long-term safety of this relatively new class of biologics is yet to be determined. Thus, long-term safety data from open-label extension studies and registries, as well as head-to-head comparison amongst these biologic agents, is important to further elucidate long-term safety and efficacy profiles of the three current IL-23 inhibitors in the treatment of MTSPP.
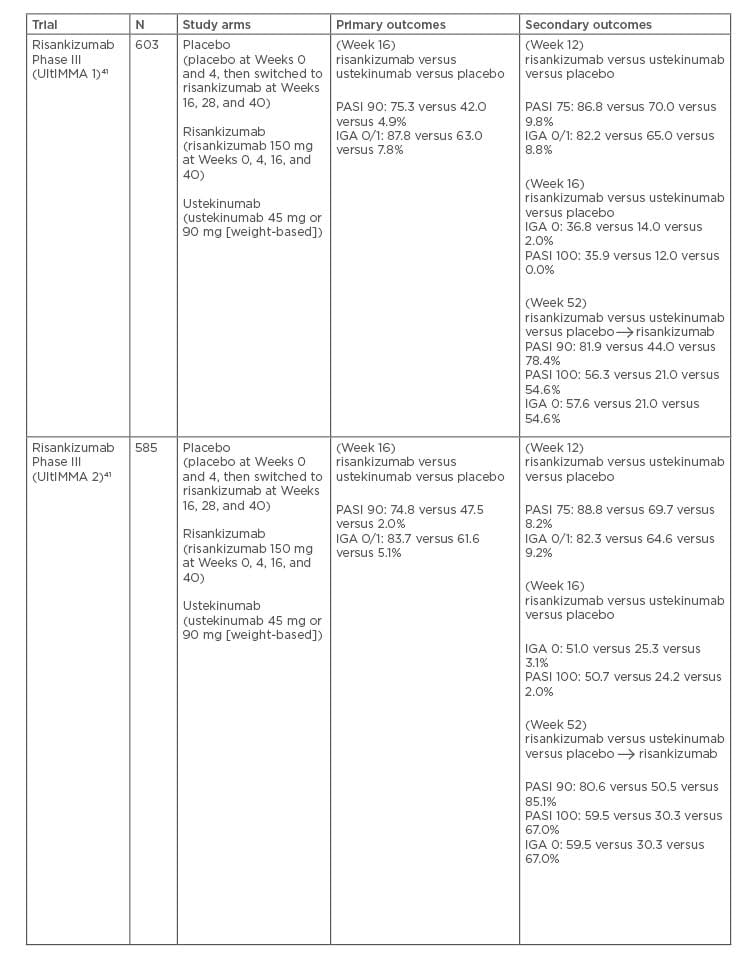
Table 3: Pivotal Phase III trials for risankizumab







