Abstract
Atrial fibrillation (AF), the most common form of arrhythmia, increases the risk of heart failure, stroke, and death. Management of AF focusses on effectively and safely controlling irregular heart rhythm, improving symptoms, and reducing complications. Early treatment of AF is important as it may improve patient life expectancy and quality of life (QoL). Current European guidelines recommend an integrated approach to AF management that involves shared decision making between patients and multidisciplinary teams of healthcare professionals to improve access to care and patient compliance. Treatment options include the use of anticoagulants, cardioversion, rate control therapies, and rhythm control therapies. Over the long term, rhythm control strategies that include antiarrhythmic drugs (AAD) and catheter ablation are the most common methods for controlling AF. The objective of this review is to highlight current European AF care pathway management recommendations and to examine the clinical, economic, and patient impact of different treatment options, including AAD and catheter ablation. While AAD have been shown to improve QoL and are affordable in the short term, treatment is moderately effective, associated with significant side effects, and can be costly long term. Catheter ablation is a highly effective therapy choice that improves patient wellbeing and is associated with a low rate of ablation-related complications. Compared to drug therapy, catheter ablation provides a significant reduction in AF burden, reduces rates of recurrence, provides a greater improvement in QoL, and facilitates long-term cost savings.
OVERVIEW OF ATRIAL FIBRILLATION MANAGEMENT
Atrial fibrillation (AF) is an irregular heart rhythm that can cause palpitations and fatigue. Based on the duration of episodes, AF can be categorised into several types: paroxysmal (occasional AF that stops ≤7 days), early persistent (AF that lasts 7 days to 3 months), persistent (continuous AF for >7 days), long-standing persistent (episodes occur for >12 months), or permanent (episodes continue and attempts to restore sinus rhythm are ceased).1,2 AF is a progressive disease: 15–20% of patients with paroxysmal AF progress to persistent over 1 year.3-5Risk factors for AF include: lifestyle factors (e.g., obesity and smoking),2,6 other comorbid conditions (e.g., obstructive sleep apnoea, high blood pressure, and heart failure),6-8 and nonmodifiable factors (e.g., older age, family history or other genetic factors, and male sex).2,7 The symptoms of AF disrupt daily life and range from mild to debilitating.9
Atrial fibrillation increases the risk of heart failure (5-fold risk), stroke (2.4-fold risk), and mortality (2-fold risk);10 however, the seriousness of AF is critically misunderstood and 45% of AF patients are unaware that AF is a life-threatening condition.11 Patients who do not experience symptoms of AF may be at greater risk of complications and disease severity due to lack of treatment. Educational and screening programmes that increase knowledge and diagnosis of AF are important tools that can reduce the risk of stroke and death in patients with AF.12,13 Early treatment of AF is important as it may improve patient life expectancy and QoL.2
The 2016 European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) guidelines on the management of AF and the 2017 Heart Rhythm Society (HRS)/European Heart Rhythm Association (EHRA)/European Cardiac Arrhythmia Society (ECAS)/Asia Pacific Heart Rhythm Society (APHRS)/Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLAECE) expert consensus statement on catheter and surgical ablation of AF recommend an integrated management strategy and individualised treatment approach based on patient preferences with the aim of improving patient wellbeing, reducing hospitalisations, and reducing mortality.1,2 The use of anticoagulants, cardioversion, rate control, and rhythm control therapies (e.g., antiarrhythmic drugs [AAD] and catheter ablation) are recommended to manage AF.2 The objective of this review is to highlight these current European AF care pathway management recommendations and to examine the clinical, economic, and patient impact of different treatment options, including AAD and catheter ablation.
CURRENT CARE PATHWAYS FOR THE MANAGEMENT OF ATRIAL FIBRILLATION IN EUROPE
The 2016 ESC/EACTS guidelines2 and the 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement1 provide guidance on the delivery of appropriate care for patients with AF, including: management of underlying cardiovascular (CV) risk factors and reducing stroke risk to improve life expectancy; electrical or pharmaceutical cardioversion when a patient is experiencing an AF episode; rate control therapies to control heart rate; rhythm control therapies, including AAD and catheter ablation to maintain normal sinus rhythm; and to improve QoL. An overview of these current care pathway management recommendations is provided in Figure 1.

Figure 1: Current care pathways for the management of atrial fibrillation in Europe. AAD: antiarrhythmic drug; AF: atrial fibrillation; AVR: aortic valve replacement; bpm: beats per minute; CABG:coronary artery bypass graft; CHA2DS2-VASc: Congestive Heart failure, hypertension, Age ≥75 (doubled), Diabetes, Stroke (doubled), Vascular disease, Age 65–74, and Sex (female); HF: heart failure; LVEF: left ventricular ejection fraction.
Adapted from 2017 HRS/EHRA Consensus Statement1 and 2016 ESC Guidelines.2
Studies indicate that screening to identify unknown AF can identify 1.4% of the population ≥65 years of age with previously undiagnosed AF.14 The 2016 ESC/EACTS guidelines provide recommendations for screening for AF in at-risk populations, especially the elderly and stroke survivors.2 Opportunistic screening for AF is recommended by pulse taking or ECG rhythm strip in patients >65 years of age.Recommendations for screening for AF in patients with transient ischaemic attack or ischaemic stroke incudes short-term ECG recording followed by continuous ECG monitoring for ≥72 hours. Patient-operated ECG devices, and continuous ECG monitoring using skin patch recordings have been validated for detection of paroxysmal AF, while newer technology advances (e.g., smartphones, smart watches) are currently under investigation for their potential role in detecting silent, asymptomatic AF.2
Initial Atrial Fibrillation Patient Care Pathway Management
Following the diagnosis of AF, guidelines recommend an integrated and structured approach to patient care and AF management that involves patients and multidisciplinary teams of cardiologists and electrophysiologists, nonspecialist healthcare professionals (e.g., primary care physician, registered nurse), and allied healthcare professionals (e.g., dietician, medical technologist), and places the patient in a central role in decision-making.2 The key aims of integrated management of AF disease and collaborative decision making are to tailor management to patient preferences, reduce hospitalisations, improve adherence to long-term therapy, and to reduce mortality.
Because the presence of CV risk factors often exacerbates AF,2 and AF is associated with an increased risk of stroke compared to patients in sinus rhythm,10 the initial therapeutic goal for AF is to treat any underlying CV conditions and reduce the risk of stroke.2 The following CV risk factors and key disease-related complications are commonly assessed: stroke, heart failure, hypertension, valvular heart disease, diabetes, obesity, obstructive sleep apnoea, and chronic kidney disease.2 To achieve CV risk reduction, lifestyle changes and the treatment of underlying CV condition are recommended.2 Stroke prevention with oral anticoagulants (e.g., warfarin, dabigatran, rivaroxaban, apixaban, and edoxaban) is recommended in patients at risk of stroke.2 These AF patient care pathway management strategies aim to approve patient QoL, autonomy, social functioning, and life expectancy.2
The CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 [doubled], diabetes, stroke [doubled], vascular disease, age 65–74, and sex[female]) score and the HAS-BLED (hypertension, abnormal renal/liver function [1 point each], stroke, bleeding history or predisposition, labile INR, elderly [>65 years], drugs/alcohol concomitantly [1 point each]) score are used for evaluating stroke and bleeding risk, respectively, in patients with AF.2In patients with stroke risk factors (CHA2DS2-VASc score of ≥1 for men and ≥2 for women), oral anticoagulation is recommended.2 Guidelines recommend the reduction of modifiable risk factors (e.g., treating hypertension, reducing antiplatelet and nonsteroidal anti-inflammatory drugs) in patients with AF on oral anticoagulation.2 The guidelines also make recommendations for occlusion or exclusion of the left atrial appendage for the prevention of stroke. Anticoagulation should be continued in at-risk patients with AF for stroke prevention and left atrial appendage occlusion may be considered for stroke prevention in patients with AF and contraindications for long-term anticoagulation treatment. Further research is needed to inform the best use of left atrial appendage occlusion devices (e.g., Watchman™), especially in patients who are unsuitable for oral anticoagulation or in patients who suffer a stroke on oral anticoagulation.
Rate and Rhythm Control Strategies in Atrial Fibrillation Patient Care Pathway Management
Atrial fibrillation care pathway management includes rhythm control therapy to restore sinus rhythm during an episode of AF and rate and rhythm control therapies in the long term. Rhythm control therapies include electrical and pharmacological cardioversion with the type of cardioversion chosen dependent on haemodynamic stability, presence and type of structural heart disease, and patient choice.2 Long-term rhythm control therapies include pharmacological (i.e., AAD), interventional (i.e., catheter ablation), or surgical (i.e., surgical ablation) options. Rhythm control strategies that include AAD and catheter ablation are the most common long-term methods for controlling AF, effectively preventing recurrence in as many as 94% of patients over the course of 1 year.2,15-20 The choice of an alternative rhythm control therapy requires patient involvement, consideration of patient preferences, and informed decision-making with a multidisciplinary team of healthcare professionals, should the first rhythm control strategy fail.2 Patients who experience recurrence of symptomatic AF while on AAD or after catheter ablation may choose to receive treatment with a different AAD, undergo catheter ablation again, receive hybrid therapy (i.e., combining AAD with ablation), or start rate control therapies to control AF rate or symptoms.2
Several therapies previously used to treat AF are no longer recommended or are only recommended for use in select patient populations.2 Implantable cardioverter defibrillators are not indicated for rhythm control of AF and pacemakers are only recommended for use in patients with sick sinus syndrome and/or bradycardia.
IMPACT OF ANTIARRHYTHMIC DRUG THERAPY IN THE MANAGEMENT OF ATRIAL FIBRILLATION
Overview of Antiarrhythmic Drug Therapy
AAD therapy is an integral part of maintaining sinus rhythm after cardioversion; AAD act to suppress the firing of or depress the transmission of abnormal electrical signals.2 Several Class I (sodium channel blockers) and Class III (potassium channel blockers) AAD are available for rhythm control, including, Class 1A: disopyramide and quinidine; Class IC: flecainide and propafenone; and Class III: amiodarone, dronedarone, dofetilide, and sotalol. In the 2016 ESC/EACTS guidelines, flecainide, propafenone, dronedarone, and sotalol are recommended (Class 1A recommendation) for preventing symptomatic AF in patients with normal left ventricular function and without pathological left ventricular hypertrophy.2
Choice of AAD is primarily guided by safety considerations, including absolute or relative contraindications, risk factors for adverse events (AE) such as onset of new arrhythmia or exacerbation of existing arrhythmia and effects outside the heart, factors that influence drug disposition (e.g., patient age and renal or hepatic function), and patient preference.2 Guidelines recommend placing patients in the central role in the decision-making process to improve patient compliance and reduce the clinical consequences of AF.2
Clinical Impact of Antiarrhythmic Drug Therapy
AAD is relatively safe and moderately effective at maintaining normal sinus rhythm. Rates for maintaining normal sinus rhythm with AAD at 1 year range from 33–56%;21 however, 48% of patients with AF are not well managed on AAD.22 The toxicity profile of AAD is varied and frequently includes drug-induced arrhythmia in 2–4% of patients and AE leading to treatment discontinuation in 12–19% of patients.2,21,23 Diarrhoea, nausea and vomiting, headache, and dry mouth are commonly experienced AE associated with AAD. Treatment withdrawal rates as a result of AE vary according to medication class (Class IA: 19%, Class IC: 12%, and Class III: 13%).21Reported event rates for stroke, heart failure, and mortality are low; however, the potential benefits of AAD in reducing these events are yet to be established.2,21,24
The Impact of Antiarrhythmic Drug Therapy on Patients
AAD therapy is effective at reducing AF recurrences,21 controlling symptoms of AF, and improving patient QoL.25 In the A4 study, paroxysmal AF patients (N=112) treated with AAD showed a 13% reduction in symptom frequency (p=0.002) and a 38% reduction in symptom severity (p<0.0001) as measured by the AF Symptom Frequency and Severity Checklist.25-28 Improvements in QoL were experienced at 1 year after AAD initiation as demonstrated by an increase in SF-36, including a 14% increase in the physical component (p=0.001) and 18% increase in the mental component (p=0.0001) subscales.
Economic Impact of Antiarrhythmic Drug Therapy in Atrial Fibrillation
Several studies have shown that AAD are cost effective. Treatment costs for AAD are offset by reductions in rates for AE, stroke, and mortality.29-31 Although the initial cost of AAD treatment is relatively low, the length of treatment is indefinite and the cumulative cost of AAD increases annually. For example, one French cost analysis study found that the cumulative cost of AAD in paroxysmal AF patients treated with two AAD increased 28% annually, over 9 years.32 Table 1 illustrates the potential treatment costs for managing patients with AF using AAD in France, Germany, Italy, Spain, and the UK, based on current efficacy and event rates for AAD and unit costs reported in the literature. The cost of AAD therapy is influenced by its toxicity level and effectiveness in restoring sinus rhythm and reducing the risk of AF-related consequences, such as stroke and heart failure.31,33-43
IMPACT OF CATHETER ABLATION IN THE MANAGEMENT OF ATRIAL FIBRILLATION
Overview of Catheter Ablation
Catheter ablation is used to create small scars on targeted parts of heart tissue that block the abnormal electrical signals causing the arrhythmia in AF.1,2 Ablation strategies commonly include isolation of the pulmonary veins and creation of specific lines of lesions within the left atrium.1 Key considerations for treating patients with catheter ablation include: type of AF, presence of structural heart disease and other comorbidities, risk of complications, patient preference, degree of symptoms, candidacy for alternative therapies (e.g., rate control, AAD), patient age, and frailty.1
Clinical Impact of Catheter Ablation
Prior to 2012, long-term rates of freedom from atrial arrhythmia were reported to be 54.1% in paroxysmal AF patients and 41.8% in nonparoxysmal AF patients.44 More recently, higher rates of freedom from atrial arrhythmias have been reported in clinical studies at 1 year after a single procedure with advanced catheter ablation technology in paroxysmal (84–94%)15-20 and persistent (59–83%)15,18,45-48 AF patients. Studies similarly show that a single catheter ablation procedure effectively maintains sinus rhythm in eligible patients with AF and heart failure (38–75%)49-51 and in elderly patients ≥75 years of age (78%).52
Catheter ablation is associated with a low risk of AE. Up to 10% of patients may experience a complication.2 Potentially life-threatening but manageable complications may occur in 2–3% of patients (i.e., periprocedural death, oesophageal perforation or fistula, periprocedural stroke [including transient ischaemic attack or air embolism], or cardiac tamponade).2 Complications of an unknown significance (i.e., asymptomatic cerebral embolism and radiation exposure) range from 5–20%.2
The relative safety of catheter ablation was reconfirmed in the largest randomised control trial examining catheter ablation in AF, the CABANA trial. In this trial, complications were rare; the most serious AE reported was cardiac tamponade (occurred in 0.8% of the study population) and there was no incidence of atrial oesophageal fistula in >1,000 symptomatic AF patients.53 Catheter ablation also normalises the incidence of AF-related consequences during long-term follow-up.54Using data from a large study derived from a prospective registry, compared to matched controls without AF, AF patients who underwent ablation had similar rates of death (p<0.0001), stroke (p<0.0001), Alzheimer’s dementia (p<0.0001), senile dementia (p<0.0001), and vascular dementia (p=0.001) at 1 year and 3 years.
The Impact of Catheter Ablation on Patients
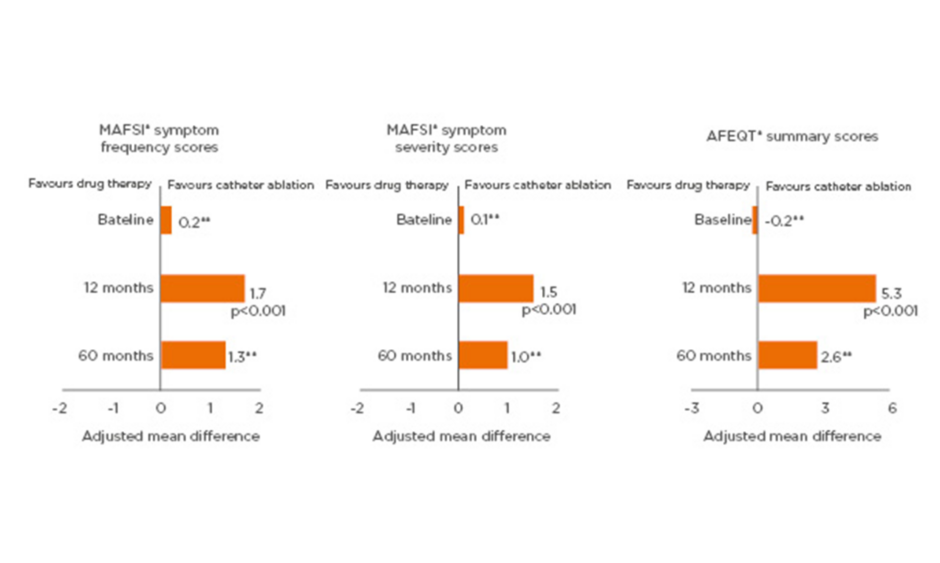
Catheter ablation is highly effective at controlling AF symptoms and significantly improves patient QoL. In the CABANA trial (N=2,204 symptomatic AF patients), improvements in symptoms and QoL after catheter ablation of AF were demonstrated at 12 months and maintained at 60 months, as demonstrated by reductions in the Mayo Atrial Fibrillation-Specific Symptom Inventory (MAFSI) scores and improvements in Atrial Fibrillation Effect on Quality of Life (AFEQT) and SF-36 physical and mental component summary scores.26
Economic Impact of Catheter Ablation
Several studies have shown that catheter ablation of AF is cost-effective when benefits are maintained over the medium to long-term, with improved QoL and reduced cost of follow-up treatment identified as key drivers influencing cost.31,41,55-59 European data on medical visits before and after catheter ablation are limited; however, evidence outside of Europe shows that catheter ablation reduces the need for unplanned medical visits compared to before ablation, with reductions of <80% at 2 years.60 Table 1 illustrates the potential treatment costs for managing patients with AF with catheter ablation in France, Germany, Italy, Spain, and the UK, based on current efficacy and event rates for catheter ablation and unit costs reported in the literature. Improved efficacy and reductions in unplanned medical visits after catheter ablation can lead to reduced costs for managing AF.31,33-41
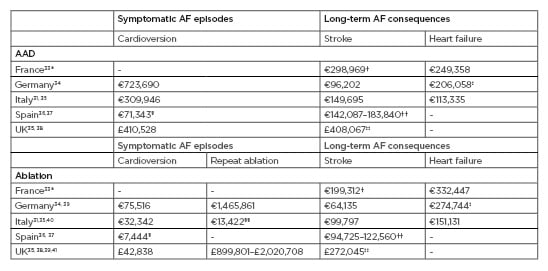
Table 1: Potential treatment costs for managing patients with atrial fibrillation with antiarrhythmic drug therapy and catheter ablation in Europe. Costs are estimates for 1,000 patients, based on efficacy and event rates for AAD and ablation reported earlier, and unit costs reported in the literature. Unit costs were inflated to 2019 Euros.42 *based on mean per patient per event costs in AF patients; †cost reported is a mean per patient per event of stroke, transient ischaemic attack, and systemic embolism; ‡assumes costs for hospital admissions for pacer implantation represents heart failure hospitalisation; §electrical cardioversion only; ††includes fatal ischaemic stroke, and mild, moderate, and severe ischaemic stroke events; ‡‡includes intracranial haemorrhage, haemorrhagic stroke, and ischaemic stroke; §§based on mean per patient per year cost in AF patients. AAD: antiarrhythmic drug; AF; atrial fibrillation.
IMPACT OF CATHETER ABLATION COMPARED TO DRUG THERAPY IN MANAGING ATRIAL FIBRILLATION
Clinical Impact of Catheter Ablation Compared to Drug Therapy
The clinical efficacy of catheter ablation compared to drug therapy has been assessed in several global trials, including the CABANA,53,61 CASTLE-AF,62 and ATTEST63 trials. These trials show catheter ablation is more effective in preventing recurrence, complications, and progression of AF than drug therapy, with a similar rate of AE. In the CABANA trial, a significant 48% improvement in freedom from atrial arrhythmia over 4-year follow-up period was demonstrated with catheter ablation, compared to drug therapy (hazard ratio [HR]: 0.52; 95% confidence interval [CI]: 0.45–0.60; p<0.001).48 Catheter ablation was associated with reduced incidence of AF complications including death, stroke, and cardiac arrest versus no treatment.53,61 The composite endpoint for death or CV hospitalisation was statistically different between the catheter ablation group versus the drug therapy group (51.7% versus 58.1%, HR: 0.83; 95% CI: 0.74–0.93; p=0.001). In the CASTLE-AF trial (N=363), which included patients with AF and heart failure, >60% of patients who underwent catheter ablation maintained sinus rhythm compared to ~25% of those on drug therapy at 1-year follow-up (p<0.001).62 Catheter ablation was associated with a significant improvement of ≤47% in survival, free from death, or heart failure hospitalisation compared to drug therapy over 5 years’ follow-up.62 In ATTEST (N=255), patients with paroxysmal AF who underwent catheter ablation were 10-fold less likely to progress to persistent AF, compared to the cohort using AAD (HR: 0.11; 95% CI: 0.02–0.48; p=0.0034).63 Studies report a similar frequency of AE when treating patients with catheter ablation or drug therapy; however, the types of events are often specific to the treatment strategy.53,62,64
Patient Impact of Catheter Ablation Compared to Drug Therapy
A significantly greater improvement in patient QoL with catheter ablation of AF compared to drug therapy has been demonstrated in two randomised controlled trials: the CABANA,26 and the CAPTAF64 trials. In CAPTAF, SF-36 QoL summary scores measuring general health, physical health, and mental health were significantly higher among those patients treated with ablation versus drug therapy at 1 year (between-group differences: physical health: 8.9 points, p=0.003; mental health: 6.1 points, p=0.02; physical health: 5.3 points, p=0.02).64 In CABANA, MAFSI frequency and severity scores, and AFEQT summary scores, were more favourable in the catheter ablation group than the drug therapy group at 1 year and maintained over 5 years (Figure 2).26
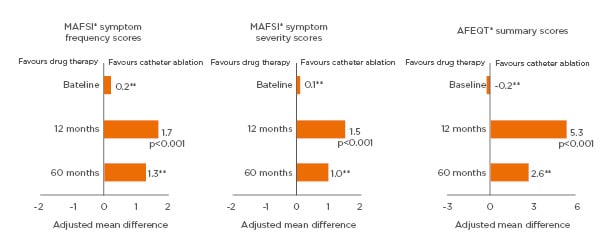
Figure 2: Significantly greater improvement from baseline in quality of life with catheter ablation than with drug therapy at 1 year and maintained over 5 years among atrial fibrillation patients in CABANA.
*As measured by the MAFSI and AFEQT questionnaire. **Statistical significance not reported.
AFEQT: Atrial Fibrillation Effect on Quality of Life; MAFSI: Mayo Atrial Fibrillation-Specific Symptom Inventory.
Adapted from Mark et al.26
Economic Impact of Catheter Ablation Compared to Drug Therapy
Studies indicate that catheter ablation is cost-effective compared to AAD for the management of AF.65In a recent UK database analysis comparing 1-year resource utilisation after catheter ablation to that with AAD, catheter ablation was associated with significantly less resource utilisation than AAD over 1 year (including a 3-month blanking period), a 51% relative reduction in CV-related outpatient visits (p<0.001), and 38% lower inpatient admissions for heart failure (p=0.0318).65 Although economic studies comparing ablation to AAD are limited across European counties, several economic analyses show that ablation is cost-effective compared to AAD due to its greater clinical effectiveness.31,41,55-59 A French cost analysis examining the cumulative costs of paroxysmal AF treatment over 10 years showed that costs become favourable for catheter ablation at 5 years after the initial ablation procedure when compared to AAD, despite the larger initial investment.32
FUTURE DIRECTIONS
The 2016 ESC/EACTS guidelines and the 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement highlight key areas for future research, which will help establish the optimal strategies to be used in future recommendations and patient care pathway strategies for the management of AF.1,2 Although CABANA and CASTLE-AF examined long-term outcomes of catheter ablation ≤5 years, further research is needed to examine long-term outcomes beyond 5 years. Further research is needed to understand key elements of integrated healthcare management teams, oral anticoagulation therapy, rhythm control outcomes, progress in rhythm control therapy, and recurrence of AF after catheter ablation. For example, with regards to an integrated healthcare management team, further research is required to understand whether a multifunctional team approach, including general cardiologists, electrophysiologists, surgeons, and other specialists, leads to better outcomes for AF patients than care delivery through isolated pillars of care, and to understand the optimal role for each member of the care delivery team. For oral anticoagulation therapy, it is unclear if a patient who has subclinical or no AF after successful catheter ablation needs oral anticoagulation, and whether there are patients who can safely discontinue oral anticoagulation therapy. Research is also foreseen to understand progress in rhythm control therapy and to determine the clinical and economical value of technological innovation for both drug therapy and ablation. Finally, there is limited data on the optimal treatment strategy in patients who experience recurrence of AF after catheter ablation and whether patients should receive a repeat catheter ablation, surgical ablation, AAD, or hybrid therapy (i.e., combining AAD with ablation).
CONCLUSION
This review promotes greater awareness and understanding of the current care pathways for the management of AF in Europe and highlights the current evidence for the clinical and economic impact of AAD and catheter ablation. Overall disease management of AF focusses on controlling the irregular heart rhythm, improving symptoms, and reducing key complications based on shared decision-making between healthcare professionals and patients. Treatment guidelines recommend rhythm control therapies to maintain normal sinus rhythm in patients with AF. Studies demonstrate that AAD therapy is moderately effective and is associated with treatment withdrawals, but it has been shown to improve QoL and is affordable over the short term. Catheter ablation is more effective at reducing symptom burden than drug therapy, is associated with lower rate of recurrence, provides a significantly greater improvement in QoL, and is less costly over the long term.