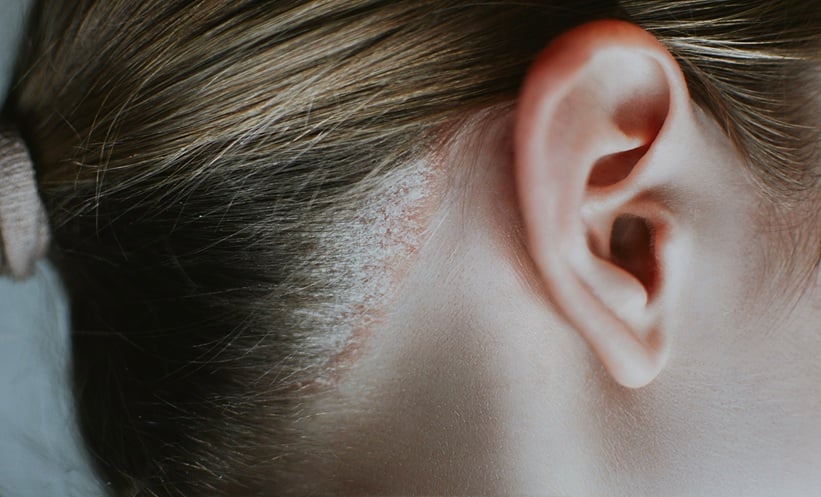
SCALP psoriasis presents a significant treatment challenge, as topical therapies are often cosmetically unacceptable for many patients. Tildrakizumab, an anti–interleukin (IL)-23 p19 antibody, is approved for treating adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy. However, initial phase 3 trials that supported the approval of tildrakizumab were not specifically designed to assess its efficacy in difficult-to-treat areas like the scalp.
To address this gap, a phase 3b, randomised, double-blind, placebo-controlled study (NCT03897088) was conducted to evaluate the efficacy and safety of tildrakizumab in patients with moderate-to-severe scalp psoriasis. At week 16, significantly more patients receiving tildrakizumab 100 mg achieved the primary endpoint – an Investigator Global Assessment modified 2011 (scalp) score of 0 or 1 with at least a 2-grade improvement from baseline – compared to those on placebo. Efficacy was consistent across subgroups, regardless of prior tumour necrosis factor–α inhibitor use or body weight, and no new safety concerns were identified.
Given psoriasis’s chronic, relapsing nature, maintaining long-term treatment response is essential. The week-52 analysis demonstrated sustained efficacy and safety of tildrakizumab for scalp psoriasis. Response rates for the primary endpoint improved from 49.4% at week 16 to 62.9% at week 52. Key secondary endpoints also showed continued improvement, with the majority of responders at week 16 maintaining their results at week 52. Patients initially receiving placebo experienced notable improvement after switching to tildrakizumab.
While other biologics like secukinumab and etanercept have demonstrated short-term effectiveness, long-term data remain limited. This trial offers the first dedicated evidence of sustained efficacy for an IL-23 inhibitor specifically for scalp psoriasis. Notably, patients remained on a consistent 100 mg dose throughout the 52 weeks, allowing clear assessment of long-term effects.
This study’s strengths include its stringent outcome measures and long-term design. However, it excluded patients with predominantly scalp involvement and minimal whole-body psoriasis, limiting generalisability. Nevertheless, real-world data from a German multicentre study suggest that the efficacy of tildrakizumab extends beyond clinical trial settings, offering promising prospects for patients with scalp psoriasis.
Reference
Sofen HL et al. Efficacy and safety of tildrakizumab for the treatment of moderate-to-severe plaque psoriasis of the scalp: Week 52 results from a phase 3b, randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2024;DOI:10.1016/j.jaad.2024.12.018.