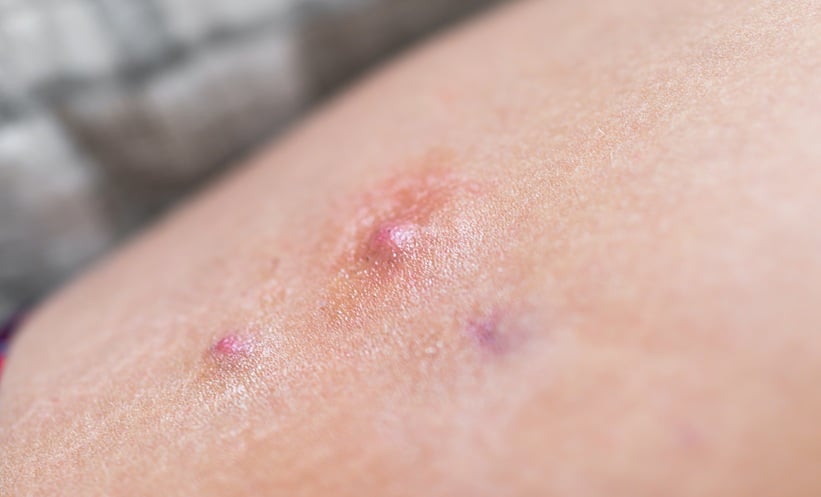
LONG-term data reveal that bimekizumab, a dual IL-17A and IL-17F inhibitor, provides sustained symptom control for patients with hidradenitis suppurativa (HS). Presented at the 14th Conference of the European Hidradenitis Suppurativa Foundation (EHSF) 2025, the findings show that after 2 years of treatment, 83.4% of patients remained flare-free, while more than half (53.1%) had mild disease, compared to 0% at baseline.
Bimekizumab is the first and only approved biologic targeting IL-17A and IL-17F, offering a novel approach to treating moderate to severe HS. These latest results reinforce its durability as a treatment option for this chronic, painful skin condition.
Among patients who initially responded to bimekizumab at 1 year, more than 85% maintained their response through 2 years, with 86.9% achieving HiSCR75 (at least 75% reduction in abscess and inflammatory nodule count). Additionally, clinician assessments showed a shift in disease severity, with the proportion of patients with severe HS dropping from 87.4% to 20.4%, while those with mild HS increased from 0% to 53.1%.
These 2-year data demonstrate bimekizumab’s ability to provide sustained disease control, meaning a shift towards mild disease characterized by the absence of draining tunnels.
Further data from the BE HEARD EXT trial on bimekizumab’s safety and efficacy profile are required.
Reference: UCB. BIMZELX[®]▼(bimekizumab) two-year data at EHSF 2025 demonstrate sustained disease control in hidradenitis suppurativa (HS). February 12, 2024. Last accessed: February 21, 2024. Available at: https://www.ucb.com/newsroom/press-releases/article/bimzelxrvbimekizumab-two-year-data-at-ehsf-2025-demonstrate-sustained-disease-control-in-hidradenitis-suppurativa-hs.