Abstract
Peak sporting performance requires optimal levels of health and fitness. Rhinitis, with its proven detrimental effects on sleep and mood, and its association with asthma, can clearly compromise athletic ability. Nasal health is therefore of key importance to the athlete. While not a limiting factor in a single exercise effort, the effects of nasal dysfunction can have repercussions in the post-exercise recovery period. Furthermore, it is linked with the development of asthma and may increase susceptibility to upper respiratory tract symptoms. This review aims to investigate the physiology of the nose during exercise, examine the relationship between exercise and nasal dysfunction, and consider the impact that dysfunction may have on an athlete. Lastly, the authors describe the diagnosis and treatment of rhinitis in athletes.
PREVALENCE OF RHINITIS IN ATHLETES
The reported prevalence of allergic rhinitis (AR) in the normal population differs from country to country. In a study using the Allergic Rhinitis and its Impact on Asthma (ARIA) definition for the European population, the prevalence was found to be around 25% and ranged from 17.0–28.5%.2 Moreover, an increasing trend in the prevalence of AR was observed over the last decades of the past millennium, but in the last 25 years this trend seems to have tailed off.3,4 The prevalence of non-allergic rhinitis (NAR) in the normal population is not as well studied as AR, but it has been reported to account for 17–52% of all cases of rhinitis in adults.5,6
Rhinitis in athletes has frequently been studied in combination with asthma and, although there are many literature estimates of prevalence in the athletic population, the estimates of frequency of rhinitis vary widely, ranging from 27–74%.7-9 This variation might suggest heterogeneity of either the population or sampling methods. Recently, a systematic review examined the prevalence of rhinitis in athletes in three separate subgroups (land, water, and cold air) according to the environment where they spent the most training hours.7 After doing so, the range narrowed and was easier to interpret: track and field athletes were not affected by rhinitis significantly more than the general population, regardless of whether they were endurance or sprint specialists;10 contrary to this, 48.6% of the athletes who spent training hours in cold environments reported rhinitis, with the distinctive, and often severe, symptom being rhinorrhoea (96%).11
There are several studies examining the prevalence of rhinitis in swimmers.12-15 Surda et al.12 studied a large cohort in which rhinitis was reported significantly more often in elite swimmers (45%) than in non-elite swimmers (31%), non-swimming athletes (32%), and controls (24%). AR prevalence was similar in all groups, but the prevalence of NAR was significantly higher in elite swimmers (33%) and non-elite swimmers (22%) compared to non-swimming athletes and controls.12 An interesting new finding was that while AR prevalence was not affected, NAR prevalence increased in these patients. This study’s findings do not support those of a Dutch study on 2,359 swimming children aged 6–13 years, which indicated that sensitisation to common allergens may be correlated with increased swimming frequency, especially during pool attendance in the first 2 years of life.16 This is in agreement with a prospective longitudinal study in British children that did not show an increased risk of developing allergic symptoms or asthma in swimming children.17
PATHOPHYSIOlOGY
The acute effects of exercise on the nose have been well delineated: vasoconstriction of the capacitance vessels results in a measurable increase in nasal volume.18 In aerobic exercise, nasal minute ventilation increases absolutely but proportionately contributes less than at rest, because the low resistance oral airway is used preferentially.19 The impact of repeated exercise training on nasal physiology is less well established. Many of the environments and endeavours which athletes immerse themselves in have the potential to harm the nasal mucosa. For example, an exercise which takes place in cold environments (e.g., skiers, snowboarders, ice hockey) induces glandular hypersecretion and nasal discharge in normal subjects (under parasympathetic control) and this response shows increased severity in rhinitis patients.20 Furthermore, during regular training, athletes are repeatedly exposed to allergens, cold air, and pollutants, and these can have a significant effect on their allergic diseases and respiratory physiology.21 For instance, the nasal obstruction of rhinitis patients shifts the pattern of nasal breathing to oral breathing, increasing the exposure of lower airways to allergens, pollutants, or other adverse environmental factors. Some studies have shown that nasal breathing significantly reduced exercise-induced asthma,22 due to the nose’s role in humidifying inspired air.23
There is still a debate about the causative factor of rhinitis in elite swimmers. The most commonly accepted theory is that repeated exposure to chlorination byproducts, such as trichloramines (potent oxidants known to disrupt epithelial tight junctions), can facilitate the penetration of allergens or pollutants and the migration of inflammatory cells across the epithelial barrier.13,24-27 It has been reported that 1 hour spent in a chlorinated swimming pool was sufficient to increase airway epithelium permeability in swimmers, whereas no change was observed after attending a copper-silver disinfected pool.28 Compared with other sport athletes, swimmers demonstrate specific features in their breathing patterns characterised by a low breathing frequency but high tidal volume, which may favour a hypothesis of significant mechanical stress to the airways.29 Fornander et al.30 studied indoor swimming pool personnel and reported that 17% of the subjects suffered with airway symptoms. This difference in prevalence might have shed more light on the significance of other factors like high minute ventilation.
Major rhinosinusal problems experienced by boxers are certainly of traumatic origin. The so called ‘boxer’s nose’ is typically the result of an osteo-cartilaginous nasal fracture with detachment of the distal tip of nasal bones associated with vertical fractures of the cartilaginous septum. Moreover, recurrent trauma, often associated with an exasperated use of haemostatic pencils, can lead to significant and important alterations at the rhinosinusal mucosal level in boxers. Specifically, post-traumatic oedema, associated with the reflex glandular hyper-secretion, can induce significant alterations of the mucociliary transport system, the consequence being increased risk of rhinosinusal infections in these subjects.31
Exercise-induced changes in nasal secretions during exercise have had surprisingly little attention from researchers in the field, far less than that given to salivary markers of immune function. Early studies have confirmed that the volume of nasal secretions produced increases during submaximal exercise.32,33 There is a wealth of evidence supporting acute and chronic reductions in salivary IgA and antimicrobial protein levels during maximal exercise and heavy training periods.34-36 By extrapolation, it might be expected that nasal secretions would follow a similar pattern. However, further investigation into the adaptations in nasal mucosal immunity in response to exercise is required to make robust conclusions.
NASAL CHANGES ASSOCIATED WITH EXERCISE IN ATHLETES
A recent systematic review has well described nasal changes associated with rhinitis in athletes.37 Nasal mucosal changes triggered by sport activity can be reflected in predominant neutrophilic infiltration with reduced phagocytic activity, deterioration of olfaction, reduced ciliary beat frequency, and prolonged mucociliary transport time (MCTt).13,31,38,39 These changes can be chronic or acutely related to a strenuous training exercise, and are also influenced by the type of activity and environment. MCTt was found to be prolonged in swimmers, which can be attributed to chlorine irritation.31,38 Deterioration of MCTt and reduced ciliary beat frequency can also be observed in runners after a 20 km race. The examination of the nasal lavage obtained immediately after the competition showed increased neutrophil counts with reduced phagocytic activity.39,40 Acute nasal mucosal changes induced by strenuous exercise in the runners recovered to the baseline within 3 days of the competition. In elite swimmers, a decrease in neutrophilic infiltration and improvement of clinical symptoms were described after 2 weeks of training cessation or 30 days-use of a nasal clip.13 Several authors have studied changes in nasal patency judged by peak naval inspiratory flow before and after exercise. Interestingly, there has been no significant difference observed.13-15,38
UPPER RESPIRATORY TRACT INFECTIONS IN ATHLETES
Upper respiratory tract infections are an enormous burden to athletes. They are the most frequent reason for presentation to sports physicians and are the most common medical problem encountered at both Winter and Summer Olympics.35,41,42 The J-curve of mucosal immunity, which proposes a depression in immunity with intensive exercise, has been suggested as a model to explain the increased frequency of upper respiratory tract infections in athletes following competitions.43 This has been supported with clinical observations following extreme endurance events, with participants being up to five-times more likely to experience upper respiratory tract infections following the event than non-participating control subjects.44,45 However, studies investigating specific pathogens have failed to identify an infectious agent in as many as 50% of athletes reporting upper respiratory tract infection symptoms.46,47 This had led to a non-infectious hypothesis that supposes that many of the upper respiratory tract symptoms classically linked with infections (sneezing, blocked or runny nose, and coughing) are secondary to airway epithelial injury, cytokine release, and mucosal oedema that arise from intense exercise. AR may therefore actually predispose the athlete to the symptoms of upper respiratory tract infection, which has the associated cost to training and wellbeing.
QUALITY OF LIFE IN ATHLETES WITH RHINITIS
Active individuals with nasal symptoms suffer a considerable detriment to overall quality of life (QoL), as demonstrated by Walker et al.,48 who showed that a mixed cohort of athletes had significantly increased SNOT-22 scores when compared to controls. Left untreated, nasal disease represents a significant burden to these individuals and could potentially limit performance in competitive athletes.49,50
Unfortunately, there are only a few studies describing the impact of rhinitis on QoL.9,12,13,48,51 The most extensively studied group are swimmers.9,12,13 This was firstly described by Bougault et al.9 who showed significantly increased Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) nasal domain scores in swimmers when compared to controls. However, this difference in overall score was not deemed significant. Interestingly, the scores improved after 2 weeks of training cessation, which attests to the irritational properties of chlorine and the effects of prolonged exposure. Of note, Gelardi et al.13 also observed a significant improvement in nasal symptoms judged by visual analogue scale score after 30 days of nose-clip use. Recently, Surda et al.12 showed a significant effect of swimming on the total RQLQ scores and all subdomains except the eye. The hours spent in the pool had a significant effect on total RQLQ and some subdomains.
Similar findings were also observed in non-swimming athletes.48,51 One study that measured the impact of daily intranasal budesonide in athletes with rhinitis demonstrated significantly improved self-assessed performance scores after just 8 weeks of treatment.51 It is not known whether these improvements translate into an objective competitive gain, but they nonetheless highlight the importance of diagnosing and treating nasal disease in this population.
RHINITIS AND ASTHMA IN ATHLETES
The last decade has seen an increased understanding of the functional complementarity of the upper and lower airways as a single ‘unified airway’. As such, rhinitis and asthma frequently co-exist, with >80% of asthmatics also having rhinitis and 10–40% of rhinitics also having asthma.52 Extensive and repeated athlete studies have returned an increased prevalence of asthma, with participants in endurance sports reporting the highest rates of asthma.53,54 The last decade has seen widespread acceptance of two distinct asthma phenotypes: exercise-induced asthma and exercise-induced bronchoconstriction. The distinction lies in background respiratory function. Exercise-induced asthma implies a background of airway hyper-responsiveness that may be exacerbated by exercise. In contrast, exercise-induced bronchoconstriction implies airway hyper-responsiveness that is solely triggered by exercise. One postulated mechanism for exercise-induced bronchoconstriction is airway drying in response to hyperpnoea, with dehydration of airway epithelium leading to injury that can be demonstrated by the increased shedding of epithelial cells and the release of inflammatory mediators.52 Common sense might suggest that athletes will follow a similar pathophysiological pattern to the general population, but the confirmation of rhinitis co-existing with exercise-induced asthma and/or exercise-induced bronchoconstriction remains largely uninvestigated.
DIAGNOSIS AND TREATMENT
An ARIA document in collaboration with GA2LEN suggested a comprehensive management plan for athletes with rhinitis:1
- Early recognition and diagnosis.
- Allergy testing.
- Recognition of associated or subclinical asthma through adequate pulmonary function tests.
- Avoidance of exposure to relevant allergens (if any) and pollutants during exercise.
Treatment to improve nasal symptoms and prevent exercise-induced bronchoconstriction without affecting athletic performance while complying with anti-doping regulations.
Early recognition and diagnosis should include a thorough history check with specific focus given to the identification of the symptom-inducing trigger (e.g., allergen, infection, cold air, swimming pool, or exercise itself), anterior rhinoscopy or endoscopy, and consideration of further imaging diagnostic methods. Every athlete should be screened for allergies as a causal factor of rhinitis. This validated Allergy Questionnaire for Athletes (AQUA) is often used as a screening tool to identify athletes with allergic disease, and a score >5 has a positive predictive value of 0.94.55
State-of-the-art guidelines, such as the ARIA guidelines, provide clinicians with evidence-based treatment algorithms for chronic rhinitis. These algorithms consist of a stepwise approach based on symptom duration and severity. Primary treatments are divided into several categories: decongestants, antihistamines, chromones, antileukotrienes, anticholinergics, corticosteroids, and immunotherapy. Nowadays, the most effective treatment modalities are topical corticosteroids (intranasal corticosteroids) or a combination of topical corticosteroids and antihistamine nasal sprays depending on the presence of AR.50
Surgical intervention should only be considered if aggressive medical therapy has failed to control a patient’s symptoms. Currently, no single modality has evolved as the gold standard for the treatment of rhinitis.
The mainstay of surgical intervention targets the inferior turbinate to control prominent nasal congestion. Complications such as atrophic rhinitis or empty nose syndrome have driven practitioners away from radical turbinectomy. Minimally invasive techniques are more favourable because they have fewer complications and they preserve ciliary anatomy.50
A study by Walker et al.48 showed a high disparity between prevalence of rhinitis and use medication: 70% of active participants described one or more nasal symptoms on most days of the year, potentially illustrating a huge body of diseases within the active population. Despite this, medication was seldom-used, with over half of the active participants with regular nasal symptoms using no medication at all. The most commonly used nasal medication by athletes was a decongestant. Resorting to over-the-counter decongestants to relieve symptoms may be a latent indicator of self-medication of rhinitis by athletes. This may, in part, be because of a fear of using prescription medications that may fall foul of anti-doping regulations. However, interestingly, the current World Anti-Doping Agency’s list of prohibited medications (Table 1)56 makes no specific reference to corticosteroids that are delivered intranasally.48
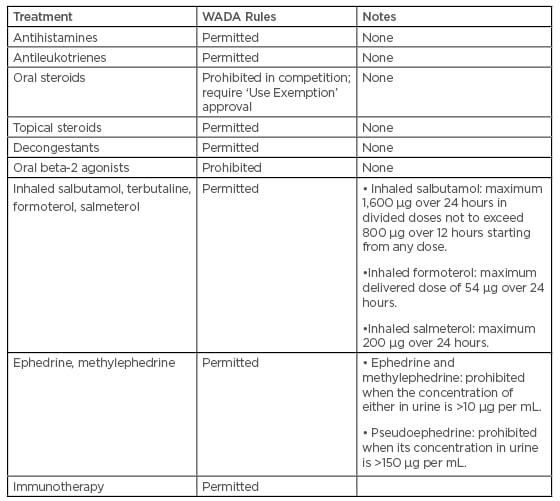
Table 1: The Prohibited List of the World Anti-Doping Agency, effective from 1 January 2019.
WADA: World Anti-Doping Agency.
DIRECTIONS OF FUTURE RESEARCH
There are several lines of inquiry to be taken to further research into the field, including but not limited to:
- Comparing allergic conditions between outdoor and indoor sports.
- Large scale studies focussing on prevalence in different types of athletes using a validated questionnaire and objective measurements.
- Studies describing the impact of rhinitis on QoL and performance in non-swimming athletes.
- Further studies examing the possibility the prophylactic treatment of rhinitis could reduce the post-exercise upper respiratory tract infection symptoms in athletes.
CONCLUSION
Nasal health is clearly of key importance to the athlete. While not a limiting factor in a single exercise effort, the effects of nasal dysfunction can have repercussions in the post-exercise recovery period. Nasal dysfunction is associated with worse sleep quality, mood scores, and QoL. Furthermore, it is linked to the development of asthma and may increase susceptibility to upper respiratory tract symptoms. Based on the evidence presented, the authors recommend that both athletes and sports physicians remain mindful of the importance of maintaining nasal health.








