Abstract
The literature reports an increased number of aphasias involving bilingual people. Dealing with bilingual aphasia requires particular attention from the diagnostic to the therapeutic phase. In this review, the authors describe the possible impairment patterns, which could be different between the two languages and be characterised by specific deficits and sometimes unexpected profiles. The role of some crucial factors in determining the observed deficits and impairment patterns is illustrated, for instance age of appropriation and proficiency. An early versus late language appropriation recruits different brain processes and hence different brain structures. In general, a greater vulnerability is observed for the late-learned languages, although a high proficiency or use and exposure appear to prevent language impairment even in the case of late appropriation. The authors also discussed the role of other intervening factors, such as emotional–motivational aspects, which could explain unusual profiles. Furthermore, language deficits specific to bilingualism, such as pathological mixing and switching and translation problems were described. In this respect, the authors underlined the fundamental involvement of cognitive control mechanisms and of the role of the brain structures associated with this. Lastly, the clinical practice issues in bilingual aphasia were outlined, underlining the need for a careful diagnosis. This should take into account the patient’s language history in order to avoid biased assessments and instead promote the setup of effective intervention programmes.
INTRODUCTION
The cases of bilingual aphasia are increasing worldwide, as they also reflect the globally increasing number of individuals speaking more than two languages (representing more than half of the population),1,2 who are referred to as bilinguals, irrespective of the number of known languages. Bilinguals differentiate one another under multiple aspects and their clinical language profiles may differ. In this review, the authors provide an overview of the different bilingual aphasia profiles and the factors associated with these different conditions, providing hints for their understanding and treatment.
In relation to the different patterns of language impairment or recovery, Paradis3 proposed, in 1977, the first structured classification: a) parallel impairment, in which the languages are similarly compromised; b) differential impairment, in which one language is more affected than the other; c) selective impairment, in which only one language is affected and the other is spared; d) blended or mixed impairment, in which there is interference between the languages and the patient cannot keep them separated; e) antagonistic, in which improvement in one language is associated with an increased impairment in the other and vice versa; and f) successive recovery, characterised by improvement in one language taking place only after the complete recovery of the other.
These impairment patterns reflect the interplay between many factors. These include first the clinical parameters that shape aphasia in monolinguals as well, such as lesion volume or patients’ age, but, crucially, also the patients’ language background. To this regard, the age at which the patients were exposed to the non-native or second language (L2) is also crucial.4,5 Age of acquisition or appropriation (AoA) is critical as it influences the way the language is represented in the brain. Neuroimaging studies in healthy bilinguals usually take the age of 6 as the cut-off to differently investigate the brain networks associated with an early versus late L2 appropriation, because around this age crucial developmental changes occur in the brain and in the learning mechanisms. Indeed, up to this age, language appropriation takes place in the form of acquisition, meaning an almost unconscious process supported by implicit mechanisms. Otherwise, late appropriation is defined in terms of learning, which instead relies on explicit processes.6-8
According to authors such as Paradis and Ullman,6-9 the role of AoA differs based on the considered language structural domain. It is particularly crucial for morpho-syntax and phonology/articulation. Internalisation of the related processes and, hence, native-like proficiency can only be achieved with early acquisition, relying on implicit mechanisms. On the other hand, lexical knowledge depends more on the degree of language use and exposure, as it is supported by explicit memory. Besides AoA, other factors also influence language mastery and related brain representation, with the chief role of proficiency.10 The following paragraphs illustrate these main factors and relate them to the impairment patterns.
PATTERNS OF LANGUAGE IMPAIRMENT IN BILINGUAL
Cases of either parallel or differential impairment are reported in many studies as the sole conditions, indicating their higher incidence with respect to the other impairment patterns. For instance, Fabbro described 20 bilinguals (AoA up to 7) with left-hemisphere damage, of whom 65% manifested parallel impairment and the remaining differential impairment. In these, either the native, first language L1 (15%), or non-native, L2 (20%), were affected the most.11 According to Paradis, the overall most frequent condition is parallel impairment, although it is underrepresented in literature, probably because it appears less appealing and therefore less worthy to be reported.6,7
Parallel impairment is frequently observed between non-native languages (for patients knowing more than two languages) when these had similar AoA, in particular when they shared the same learning modality (e.g., formal instruction).12 However, parallel impairment was also observed irrespective of AoA. For instance, Green et al.13 reported a parallel impairment between L1 and English, the L2, in patients having lived in the UK for many years, indicating the fundamental role of language use and exposure.13 The authors, however, attributed this condition to impaired control abilities, which is discussed further.
Concerning differential impairment, many patients follow either the so-called Ribot’s rule, postulating better preservation of L1,14 or the Pitres’ rule, according to which it is the most familiar language to be better preserved.15 In fact, although it is more intuitive to hypothesise greater resistance to damage for the language learned first, in many cases L1 was instead the most affected. This occurred, for instance, when the patients had a premorbid high level of L2 proficiency and frequency of use, although a recent systematic review seemed to restrict this possibility to early bilinguals.16
The patient described by Samar and Akbari17 had more preserved L2, which she learned at school, then studied at university and taught there as a teacher for 18 years. In this case, the high and deep knowledge of the language together with its constant use reduced the impairment severity. In healthy adults, the language networks appeared highly similar between L1 and L2 when proficiency is high.10 The learning method, represented by formal instruction, also had a possibly relevant role. In fact, as long as the brain lesion spares the explicit learning system and therefore the consciously learned meta-linguistic skills, formal knowledge, which relies on them, can potentially promote language recovery. This view is supported by the cases of an apparently paradoxical profile in which the patients were impaired in their native language but retained the use of an only-formally learned language, including the dead languages, such as Latin.18
Nevertheless, main exposure alone can not assure the language preservation. Impairment can indeed occur in cases of L2 learning that took place recently19 or in adulthood,20 when the brain is less prone to remodelling and language brain representation, therefore results can be less sound, hence more vulnerable to damage.
All the aforementioned language background factors indeed shape the language brain representation. Tangible information about the bilingual patients’ brain networks mainly comes from intraoperative stimulation studies. In a large-cohort study, Roux and Trémoulet21 observed that only two patients displayed solely common stimulation sites between the two languages, whereas the majority of them had both common and language-specific sites. Interestingly, language-specific sites were observed even in early bilinguals and, secondly, no additional cortical sites were found for the less-proficient language. There were similar results from a subsequent study on late but proficient bilingual patients, which further reported ‘L2-restricted zones’ in the perisylvian cortex, i.e., sites associated exclusively with L1.22 Although these findings were limited to the scouting of the affected region and its surroundings, they show that even close brain sites may be dedicated to different languages and that sometimes neither AoA nor proficiency can predict the extent of different representation between the two languages.
The depicted language brain representation leads us to suppose that a brain lesion, unless it is small and circumscribed, hardly affects one language while completely sparing the other. Nevertheless, a few cases of selective language impairment have been reported. This phenomenon was described in patients with epilepsy, with a selective postictal temporary loss of either L123,24 or L2.25 This specific type of impairment may hence have a neurofunctional rather than a neuroanatomical substrate. Changes in the normal brain electrical activity may temporarily inhibit the circuits associated with a specific language, which is recovered when the normal brain functioning is restored. In this vein, selective recovery might also result from impairment in control functions, which is illustrated in the following chapter.
The reversible inhibition of one language characterises another apparently odd condition, naming the alternating antagonism. This phenomenon is characterised by phases in which only one language seems to be accessible, whereas the other is apparently lost, and usually takes place in the immediate post-event period.26 This confirms the hypothesis that the impaired language is not lost, but inhibited, and that when this inhibition resolves, either spontaneously or throughout rehabilitation programmes, the language may recover.
Alternating antagonism is likely to take place when there is an underlying deficit in the regulation processes, which caused competition even between structurally distant languages, such as Farsi and German, as seen in the patient described by Nilipour and Ashayeri.27 The patient also manifested a successive recovery, with English (L3) recovering only after the complete recovery of the other two languages.
ROLE OF COGNITIVE CONTROL IN BILINGUAL APHASIA
Bilingualism indeed entails the need to coordinate language use to activate the proper language according to the context, while suppressing the irrelevant. This entails the constant recruitment of cognitive control functions and a certain degree of cognitive flexibility to properly shift from one language to the other.28 This could be the reason of the cognitive advantage some studies observed in bilinguals with respect to monolinguals, even in presence of aphasia.29
According to the neural convergence hypothesis,30 as bilinguals become more familiar with the new language, its brain representation converges with that of the native language. In this perspective, differences rather rely on the diverse recruitment of the cognitive control resources. Moreover, Radman et al.31 observed language improvement following stroke to be associated with increased connectivity between language areas and those devoted to cognitive control.31 However, they noticed this phenomenon was restricted to the language that improved the most, therefore suggesting that, at least in some cases (e.g., different AoA or proficiency), differences in the neural representation between the languages can actually be present.
Control deficits were observed in both parallel13,32 and differential or selective language impairment.33 Recently, many studies aimed to understand whether possible cognitive control deficits in bilingual aphasia. Some studies seem to suggest a language-specific control deficit,32 although problems in general control were also observed, and the interplay between other intervening factors, such as task complexity or lesion site were proposed to modulate the relation between language and control deficits.13,34
In their recent review on the neuroimaging of language control, Abutalebi and Green28 recapitulated their previous studies on the topic by illustrating the specific role of the brain structures associated with the language control network. These include both cortical (i.e., prefrontal cortex, dorsal anterior cingulate cortex/pre-supplementary motor area, and inferior parietal cortex, with the involvement of both hemispheres) and subcortical regions (i.e., left basal ganglia and thalamus and right cerebellum). These regions are deputed to specific functions within the language control process and lesions at their level might cause different control deficits.
Among these regions, extensive literature has highlighted the crucial role of the basal ganglia, and particularly the left (head of the) caudate and putamen, which are involved in appropriate language selection. Aglioti and Fabbro35,36 reported the case of a woman who lost her ability to speak her first language (an Italian dialect), but surprisingly began to speak Italian, which she had learned at school but rarely spoke throughout her life.35,36 She also began to speak with a strong German accent, a phenomenon known as foreign accent syndrome and described even in monolingual patients (Figure 1). This is a case of paradoxical use of one language and can be explained in light of the lesion location, which affected the left basal ganglia,39 and also stress the role of the subcortical structures in implicit memory processes, which normally support the early acquired languages.
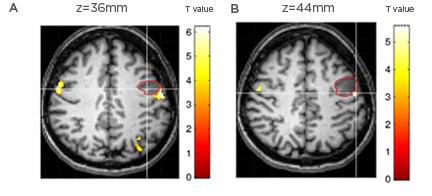
Figure 1: Foreign accent syndrome.
Foreign accent syndrome is a rare acquired motor speech variation, which has been reported in about 60 cases in literature.37 Patients suddenly exhibit a seemingly ‘strange’ accent and are perceived as having a foreign accent by listeners of the same speech community. Tomasino et al.38 reported a tumoral patient developing foreign accent syndrome following a small and circumscribed lesion in the left precentral gyrus. The patient, an Italian native speaker, developed altered speech rhythm and melody. During pronunciation of words and pseudowords in fMRI tasks, the patient showed a hyperactivation, compared to the control group, in areas around the pre/postcentral gyrus corresponding to those involved in phonation (i.e., larynx motor area). The fMRI cluster related to mouth (A) and tongue (B) movements located behind the patient’s lesion (indicated by the red circle).
For the role the basal ganglia plays in implicit memory processes, a lesion at this level is likely to predominantly affect the mopho-syntactic processes. This was reported, but even for late-learned languages of high proficiency, for which even these linguistic processes could have become automated.40 This result indicates the crucial role proficiency may have in promoting language representation reshaping throughout life.
An illustrative case of the association between lesion site and impaired language was described by Moretti et al.41 The patient manifested impairment in the native language following an infarct in the left caudate nucleus and then, when a lesion affected the left frontotemporal cortex, she developed deficits in her late-learned L2. This supports the predominant cortical representation of languages learned through explicit processes, although an opposite trend was also described.42
OTHER CLINICALLY RELEVANT FACTORS
Recovery patterns may also be modulated by factors other than AoA, proficiency, and cognitive control deficits (see Figure 2 for an overview). Bilinguals learning a new distant language try to adopt the same L1 processes, but when these turn out to be unsuitable, they need to develop new processes (assimilation-accommodation processes).43 Evidence is however lacking regarding the possibly greater impairment in L2 when structurally distant from L1.16
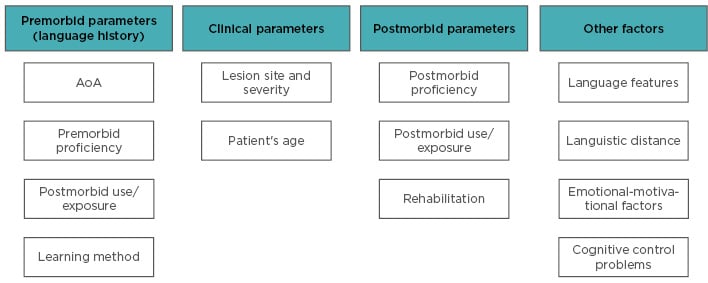
Figure 2: Overview of the factors contributing to the different language recovery patterns.
AoA: age of acquisition or appropriation.
Recorded difficulties reflect the cognitive demands required to process a given language, for instance when reading a transparent versus opaque language. This point is tricky as these differences might bias the diagnosis between the languages,44 and therefore call for a language assessment respectful of language complexity.
Other factors contributing to the definition of a given impairment pattern include the language spoken in the environment, namely in the hospital, in the period immediately following the clinical event. This factor can also be relevant from the rehabilitation viewpoint, as lower-than-expected improvements in the treated language were attributed to the fact that the patient was constantly exposed to another language outside the rehabilitation setting.45
Finally, even the affective factors can assume a fundamental role. Emotionally relevant episodes may induce the release of the apparently lost language46 and the willingness to recover a given language may actually prompt its improvement.47
SPECIFIC LANGUAGE DEFICITS IN BILINGUAL APHASIA
Differently from monolinguals, bilingual patients may develop deficits characterising specifically the bilingual condition and concerning the reciprocal use of the two languages. These symptoms include mixing (i.e., recourse of words or other elements of one language during the use of the other), switching (e.g., shift from one language to the other), and problems in translation.
Frequently, these events arise from lesions in the mentioned areas involved in regulating the proper language use,48,49 with greater interference frequently observed between two structurally close languages.19,50 Fabbro et al.51 described the case of a patient with a glioma in the left prefrontal and cingulate cortices who involuntary switched to his native language, even talking to people he knew could not understand it, indicating the inability to inhibit the process.
Lesions in other areas were observed to pathologically induce or prevent language switching and include the inferior parietal lobe52 and the fronto-temporal cortex.53 Interestingly, switching was observed during direct electrostimulation of specific brain sites (Figure 3), including the white-matter tracts connecting control and language-specific brain areas.56
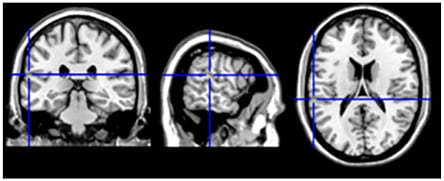
Figure 3: Switching from L2 to L1.
In neurosurgical patients, Penfield W and Roberts L54 largely documented language switching during electro-cortical stimulation mapping as an automatic mechanism that turns off one language when the other language is on. Tomasino et al.55 described involuntary language switching from L2 (Italian) to L1 (Serbian) evoked by electro-stimulation in the left superior temporal gyrus/supramarginal gyrus during awake brain surgery. The language switching site belonged to an fMRI cluster in the area Stp (in the planum temporale) which has a role in phonological processing and was found to activate for both L1 and L2 during language tasks. The language switching site (MN1 coordinaltes: x=-61, y=-30, z=18)
In some other cases, these phenomena reflect language impairment, as they occurred to compensate for anomia or other difficulties in the more impaired language.57,58 This can also be the case of translation deficits, which often reflect the general impairment in a specific language, with greater difficulty in translating from better preserved to more impaired languages than vice versa. Sometimes, this language deficit occurs selectively, despite spared ability in naming59 or in recognising translation equivalents across the languages.60 In other cases, in which naming abilities were impaired, translation processes were preserved, and further employed to recover word finding difficulties through translation from the preserved languages, therefore preventing switching.49
However, some patients were observed to paradoxically translate to the most affected language while unable to translate to the spared language,61 for instance when antagonistic recovery occurred.26 Sometimes, the patients instead manifest the compulsive tendency to translate from one language to the other, while not being able to prevent this automatic behaviour.39
ISSUES IN BILINGUAL APHASIA
Dealing with bilingual aphasia requires specific precautions from the diagnostic to the therapeutic phase. Firstly, for a proper diagnosis, it is fundamental to take into account the level of premorbid language proficiency, which was observed to be one of the most important factors in predicting the postmorbid level of deficit.62 Hence, it is fundamental to first thoroughly inspect the language history (e.g., AoA, proficiency, frequency, and context of use) by means of structured questionnaires.
Secondly, clinical assessment should ideally be performed in all the languages the patient knows, even though, especially for immigrated people, clinicians may not master the patient’s L1. When testing the different languages, it is also fundamental to take into account the structural differences between them. To this aim, Paradis and Libben developed the Bilingual Aphasia Test (BAT), now available in >70 languages, with items for each language matched in complexity and selected for their cultural adequacy.63 The battery is structured in three parts: the first inspecting the language history, the second making a comprehensive assessment of each language skills, and the last addressing specific language pairs, offering an understanding of which language was affected the most. A proper diagnosis is fundamental for setting the rehabilitation programme. Ideally, each impaired language should be treated. When this is not possible, therapists should train the language that could have more beneficial effects on the untreated language, therefore promoting cross-linguistic transfer. Although not univocally, many studies have observed that treatments focussing on the weaker language, meaning a non-native language64 or, in the case of comparable AoA, a lower-proficiency language65 are more likely to boost improvements in the untreated language.62,63 With regard to naming training, this trend can be explained in light of the revised hierarchical model by Kroll and Stewart,66 according to which L1 words are tightly linked to their correspondent meaning, whereas L2 word semantic access occurs via translation to L1. Consequently, semantic access after L2 training likely takes place by passing through the L1 lexicon, which recovers in turn. In the case of difficulty in properly regulating the language use, rehabilitating general cognitive functions is recommended first.67
Gil and Goral,68 however, qualitatively observed beneficial transfer effects following treatment in each language, in spite of the structural distance between them (i.e., Russian and Hebrew, for which transfer was poor only in writing which differs substantially between them). However, they tested the patient in the subacute phase, when spontaneous recovery was also taking place. The majority of the reported studies, however, took charge of the patients in the chronic phase and documented positive treatment effects as well. Language skill improvement and associated brain reorganisation were observed to occur just 10 days after intensive training, indicating the high brain plasticity potential even many months post-onset.69
According to Kiran et al.,70 all the parameters that have been described, such as AoA, language use, and proficiency before and after the clinical event, are relevant for the prediction of the recovery profile following treatment, although their interplay and the intervention of additional factors undermines an accurate prediction. In some instances, lack of transfer can be attributed to factors other than the treated language. These can include the choice of inappropriate rehabilitation strategies,71 the impossibility to practice the treated language outside the rehabilitation setting,45 the fact that the unimproved language had already reached its highest recovery level.72 Lastly, some partly unexpected improvements might be attributed to the willingness to recover a given language, highlighting the emotional valence the languages can take on.47
CONCLUSION
In conclusion, although it is difficult in clinical practice to concretely take account of the bilingual patients’ languages, some attempts should be made to achieve an accurate diagnosis and guarantee the most effective possible therapeutic intervention, as impairments in a given language can have relevant consequences for the patients’ life on social, affective, and working levels.








