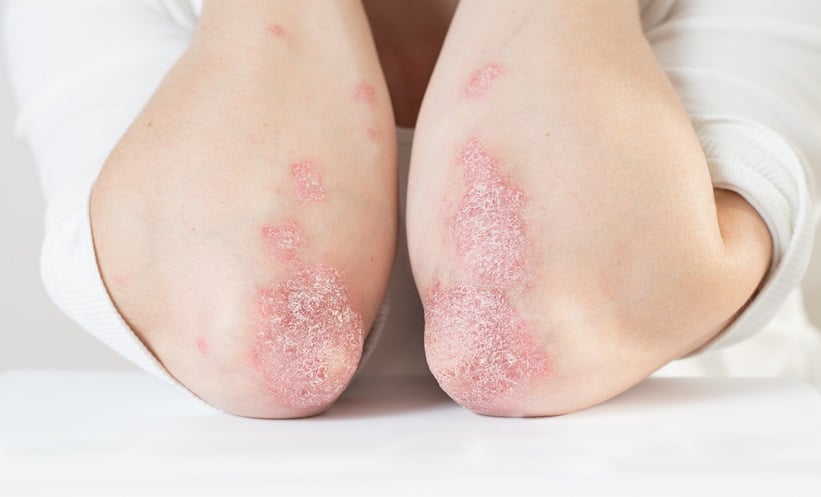
PSORIASIS, a chronic immune-mediated skin condition, poses a significant challenge for patients, with an ongoing unmet need for effective biologic treatments. To address this, a recent randomised clinical trial evaluated the safety, pharmacokinetics, and efficacy of QX004N in both healthy individuals and patients with moderate to severe plaque psoriasis in China. This two-part study offers promising insights into the therapeutic potential of QX004N.
The first phase of the trial, a single-ascending-dose study (phase 1a), involved 55 healthy participants aged 18–55. Participants received subcutaneous injections of QX004N at doses ranging from 10 mg to 600 mg or a placebo, in a 4:1 ratio. The findings revealed that QX004N was well tolerated, with linear pharmacokinetics and no serious adverse events related to the drug. Most reported side effects were mild or moderate, affirming its safety profile in healthy individuals.
The second phase, a double-blind, multiple dose-escalation trial (phase 1b), enrolled 30 patients with moderate to severe plaque psoriasis. These participants were divided into cohorts receiving QX004N doses of 150 mg, 300 mg, or 600 mg every two weeks, or a placebo, also in a 4:1 ratio. By week 12, 100% of patients receiving QX004N achieved at least a 75% improvement in their Psoriasis Area and Severity Index (PASI 75), compared to only 33.3% in the placebo group. Additionally, by week 16, all QX004N-treated patients achieved PASI 90 (90% improvement in PASI), as well as an Investigator’s Global Assessment score of 0 or 1, which signifies clear or almost clear skin.
Across both trial phases, adverse events were primarily mild to moderate, with no drug-related serious incidents reported. This consistent safety profile, coupled with the remarkable efficacy observed, highlights QX004N’s potential as a transformative treatment for moderate to severe plaque psoriasis.
While QX004N demonstrates a strong balance of safety and efficacy, offering hope for patients with this challenging condition, continued research is essential to further explore its long-term benefits and establish its place in clinical practice.
Katie Wright, EMJ
Reference
Li X et al. Safety and efficacy of anti-IL-23 monoclonal antibody QX004N for patients with psoriasis: a randomized clinical trial. JAMA Dermatol. 2024; DOI:10.1001/jamadermatol.2024.5059.