Meeting Summary
This article summarizes selected poster presentations from the 2024 Fall Clinical Dermatology Conference (FCDC), with a focus on updated data for tralokinumab in atopic dermatitis (AD) and delgocitinib cream in chronic hand eczema (CHE), and how these treatments could tackle unmet needs.
Presentations on the IL-13 receptor inhibitor tralokinumab included final data from the long-term, open-label extension study ECZTEND, of patients with moderate-to-severe AD exposed to tralokinumab for up to 6 years, as well as evaluations of the treatment’s long-term safety and efficacy in patients aged ≥65 years. An indirect comparison of tralokinumab and lebrikizumab indicated their efficacy was similarly maintained over 1 year. Real-world data for tralokinumab in AD were presented from several studies, including three interim analyses of the TRACE study, which assessed tralokinumab efficacy in AD on specific regions of the body.
Delgocitinib cream is a topical pan-JAK inhibitor recently approved in the EU for moderate-to-severe CHE in adults for whom topical corticosteroids (TCS) are inadequate or inappropriate. Several posters presented data from a pooled post hoc analysis of Phase III trials DELTA 1 and DELTA 2, demonstrating that delgocitinib led to meaningful improvements in clinical signs and patient-reported outcomes, and was well-tolerated over 16 weeks of treatment. One poster showed that twice-daily application of delgocitinib cream 20 mg/g for 16 weeks resulted in minimal systemic exposure, and two reported favorable efficacy and safety of delgocitinib compared with systemic therapies, including data from the DELTA FORCE trial. Overall, presented data supported the benefits of delgocitinib as an efficacious and well-tolerated topical treatment in a patient population that faces a high disease burden and has unmet treatment needs.
Introduction
AD, also known as eczema, is a chronic condition characterized by inflammation, redness, and skin irritation, which can significantly impact quality of life (QoL).1,2 It affects up to 10% of adults worldwide and can involve any part of the body,3,4 including the head and neck, hands, and genital region.5,6 Maintaining disease stability and preventing fluctuations is crucial for management,7 and patients with moderate-to-severe AD often require long-term treatment.
CHE is a long-term, multifactorial, inflammatory skin disease of the hands and wrists that affects around 5% of the population.8-10 Defined as hand eczema that lasts ≥3 months and relapses ≥2 times a year,9,11 CHE can manifest with crusting, scaling, fissures, and hyperkeratosis.9,11 The disease often occurs in flares and can vary greatly in severity; moderate-to-severe CHE is associated with itch, pain, and disturbed sleep, all of which can affect QoL and occupational capabilities.8,9,12,13Treatment for moderate-to-severe AD is rapidly evolving, and options include phototherapy, systemic immunomodulation or immunosuppression, systemic corticosteroids, and subcutaneously injected biologic therapies.14 However, there are currently no topical treatments specifically developed and approved for use in moderate-to-severe CHE.15 Improvements in treatment options for these conditions could help to improve management and alleviate their impact on patient QoL.
Tralokinumab in Atopic Dermatitis
Tralokinumab is a high-affinity monoclonal antibody that specifically targets IL-13, and is approved for the treatment of moderate-to-severe AD in adult and adolescent patients aged ≥12 years in the EU, Canada, and the USA, among other countries.16-18 Clinical trials and real-world data have shown that tralokinumab improves AD signs and symptoms as early as 1 month after treatment initiation, and for up to 4.5 years.19-25
Long-Term Safety and Efficacy of Tralokinumab in Atopic Dermatitis
Final long-term safety and efficacy data from the long-term, open-label extension study ECZTEND, which included patients with moderate-to-severe AD (≥12 years of age), were presented at FCDC 2024. Patients were exposed to tralokinumab for up to 1 year in the parent trials and up to 5 years in ECZTEND (N=1,672).26
Long-term use of tralokinumab was well-tolerated with no new safety signals identified. The exposure-adjusted incidence rate (IR) of patients with ≥1 adverse event (AE) in ECZTEND was lower than the initial 16-week treatment period of the parent trials. The most frequent AEs were nasopharyngitis (22.2% of patients), atopic dermatitis (21.4%), and coronavirus infection (17.9%). Serious AEs were reported in 9.0% of patients (IR: 3.54), and AEs that led to permanent discontinuation occurred in 4.5% of patients (IR: 3.54). AEs of special interest were observed at rates similar to, or lower than, the initial treatment period of the parent trials.
Tralokinumab demonstrated robust long-term efficacy with sustained improvements in AD signs, symptoms, and QoL. At Week 248, 92.9% of patients achieved ≥75% improvement in Eczema Area and Severity Index scores (EASI-75), and 66.7% achieved an Investigator’s Global Assessment (IGA) score of 0/1 (none/mild). Itch, sleep, and QoL improvements were sustained at levels equivalent to no-to-mild disease.26
Data were also presented from a post-hoc analysis of ECZTEND that aimed to determine the proportion of patients who exhibited a stable long-term response with tralokinumab. The analysis included 347 patients treated with tralokinumab ± optional topical corticosteroids (TCS) for 52 weeks in parent trials, and up to 152 weeks in ECZTEND (data cutoff: April 30, 2022).7
Results showed a high proportion (70.2%) of patients treated with tralokinumab for up to 4 years maintained stable EASI ≤7 (no-to-mild disease) across at least 80% of the days. A long-term optimal composite target of EASI ≤7 and either Dermatology Life Quality Index (DLQI) ≤5 or Itch Numeric Rating Scale (NRS) ≤4 was reached in 60.5% of patients.7
Comparison of Tralokinumab and Lebrikizumab Efficacy in Atopic Dermatitis
Both tralokinumab and lebrikizumab are monoclonal antibodies specifically targeting IL-13 that have demonstrated efficacy in patients with moderate-to-severe AD up to 52 weeks of treatment.19,27,28 However, there are no direct head-to-head comparisons of these two therapies.29
In lieu of a comparative study, data were presented from an anchored matching-adjusted indirect comparison of the efficacy of tralokinumab and lebrikizumab at Week 52 among Week 16 responders, including individual patient data from the ECZTRA 1 and 2 tralokinumab trials19,30 and aggregate data from patients in the ADvocate 1 and 2 lebrikizumab trials.29 Results showed the maintenance of efficacy after 52 weeks was comparable between tralokinumab and lebrikizumab across all endpoints. There were, however, dose-related differences. With dosing once every 2 weeks, all endpoints were numerically in favor of tralokinumab. With once every four weeks (Q4W) dosing, EASI-75 was numerically in favor of tralokinumab, yet IGA 0/1 and EASI-90 were numerically in favor of lebrikizumab.29
Real-World Patient-Reported Outcomes with Tralokinumab in Atopic Dermatitis
To evaluate the real-world impact of tralokinumab on patient-reported outcomes (PRO), adult patients with AD in the US were asked to complete a monthly online survey between February 2022–December 2024. An interim analysis of data from 107 participants was conducted after 52 weeks, and changes in PROs were presented at FCDC 2024.31
Data showed study patients with moderate-to-severe AD experienced improvements in sleep quality, itch severity, and treatment satisfaction when treated with tralokinumab, regardless of prior dupilumab experience. Improvements were greatest in the dupilumab-naïve group for all PRO measures, yet almost half of the dupilumab-experienced group also showed improvements across multiple outcomes. There was a trend in the reduction of concomitant medication use among patients treated with tralokinumab over the course of the study.31
Tralokinumab in Older Adults with Atopic Dermatitis
There are limited data on treatment with biologics among older adults (≥65 years) with AD. However, this population typically has increased safety risks and comorbidities.32 Short-term data supporting the long-term safety and efficacy of tralokinumab on adults ≥65 years have recently been published.33
At FCDC 2024, long-term safety and efficacy of tralokinumab in patients with moderate-to-severe AD aged ≥65 years was presented from a pooled, post hoc, interim analysis of ECZTEND. This analysis included patients who previously participated in a tralokinumab parent trial for up to 1 year and who were subsequently treated with open-label tralokinumab at 300 mg once every 2 weeks ± TCS for up to 3.5 years (data cutoff: April 30, 2022).32
Findings indicated that long-term use of tralokinumab in patients aged ≥65 years provided enduring control, reflecting predominantly mild-to-no disease activity, and was well-tolerated. No new safety signals were identified.32
Tralokinumab in Challenging Regions of the Body
Although AD can affect any part of the body, some regions may have a greater impact on patients’ health and well-being. For example, approximately 84% of patients with moderate-to-severe AD have involvement of the head and neck (H&N),6 which is particularly associated with social embarrassment, stigmatization, and negative impact on patients’ QoL and mental health.34 AD on the hands and feet (H&F) is also considered to have a high impact on patients due to the significant negative effects on patients’ QoL and ability to work.6,30Although rarer, presentation of AD on the genitals is often overlooked and underreported due to patients’ reluctance to discuss it with the clinician, and the lack of routine examination of this region.5 However, the presence of AD in the genital area can have a significant negative impact on pain, sleep, mood, sexual function, and personal relationships.35,36
Three posters presented at FCDC 2024 presented interim analyses of the TRACE study (data cutoff: October 15, 2023), focussing on the real-world effectiveness of tralokinumab in AD on specific regions of the body.37-39 TRACE is an ongoing, prospective, noninterventional, single-cohort study of adults prescribed tralokinumab according to national approved labels. The study enrolled patients from 167 sites from 11 countries across Europe, North America, and the Middle East, between November 2021–July 2023.37
Atopic dermatitis on the hands and feet
One interim analysis evaluated tralokinumab effectiveness in patients with H&F AD (n=493).37 Among this population, 42% of patients reported clear skin on the hands and feet after 3 months of tralokinumab, increasing to 53% at 9 months (Figure 1). Of those patients with prior experience of dupilumab, 58% showed clear skin on the H&F area at 9 months of tralokinumab. Improvements were also observed in IGA, QoL, and patient-reported ability to work in both dupilumab-naïve and dupilumab-experienced patients.37
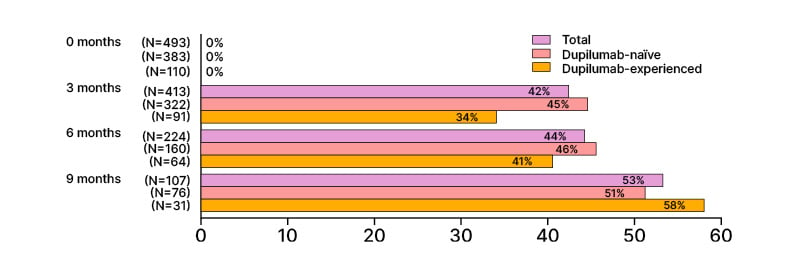
Figure 1: Percentages of patients with clear skin on the hands and feet area.37
Percentages are rounded to the nearest number. *Information on atopic dermatitis localization is not available.
Atopic dermatitis on the head and neck
Another interim analysis assessed improvements in AD on the H&N region (n=655).38 Among these patients, 3 months of tralokinumab treatment reduced the proportion with H&N involvement from 100% to 67%, and improved AD severity (IGA 0/1: 34%) and QoL (DLQI ≥6 improvement: 58%). At 9 months, the proportion of patients with head and neck involvement had reduced to 52%, with further improvements in AD severity (IGA 0/1: 57%) and QoL (DLQI ≥6 improvement: 74%) (Figure 2).38
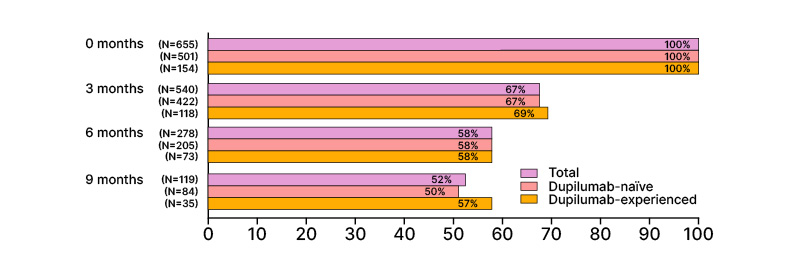
Figure 2: Percentages of patients with atopic dermatitis on the head and neck area.38
Percentages are rounded to the nearest number. *Information on AD localization is not available.
AD: atopic dermatitis.
Atopic dermatitis on the genitals
In the third interim analysis, the effectiveness of tralokinumab was evaluated in adults with AD on the genitals (n=123).39 Among these patients, 67% reported clear skin on the genitals after 3 months of tralokinumab treatment (Figure 3). Substantial improvements were also observed in AD severity, DLQI, and sleep NRS. The proportion of patients with IGA ≤2 (clear-to-mild) increased from 3% at baseline to 59% at 3 months.39
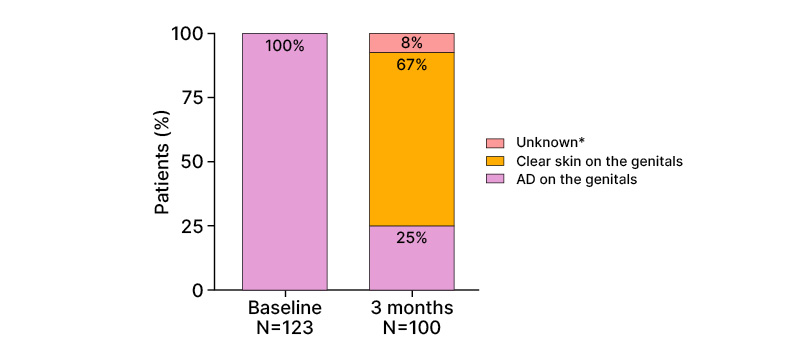
Figure 3: Percentages of patients with atopic dermatitis on the genital area.39
Percentages are rounded to the nearest number. *Information on atopic dermatitis localization is not available.
Tralokinumab Treatment Patterns in the Real World
Guidelines indicate that Q4W dosing can be considered among tralokinumab responders, yet little is known about the real-world use of this dosing interval.16,17,40 Findings were presented from a non-interventional retrospective claims study in the USA that included 1,747 adult patients, 295 (16.9%) of whom were aged ≥65 years. The mean follow-up duration was 8.3±7.7 months.40
Results showed that nearly one in three patients (29.6%) treated with tralokinumab for ≥6 months received Q4W dosing, as indicated by medication refill dates; 32.9% of these were aged ≥65 years and 29.1% were aged 18–64 years.40
Unmet Needs in Chronic Hand Eczema
Treatment of CHE is made particularly challenging by its mixed etiology, fluctuating disease course, and the need for long-term disease control. The standard first-line treatment for CHE is usually TCS; however, patients who do not respond to TCS or have TCS-related side effects have limited treatment options.11 There are no topical treatments currently available that were specifically developed for use in moderate-to-severe CHE.15,41 However, patients have indicated a preference for novel steroid-free topical alternatives for CHE treatment.42
The large, cross-sectional, multi-national CHECK (Chronic Hand Eczema epidemiology, Care, and Knowledge of real-life burden) study was conducted to generate robust, up-to-date prevalence data for CHE. It involved an online survey conducted among 60,131 adult participants from the general population in Canada, France, Germany, Italy, Spain, and the UK.10 CHE was defined as self-reported eczema on the hand(s)/wrist(s) persisting for ≥3 months or with ≥2 flares within the previous 12 months.10,11
It found that CHE was common, with an overall self-reported prevalence of 5.6% and physician-diagnosed prevalence of 4.7% in the general population.10 Among participants using TCS (without concomitant systemics or phototherapy), 60.3% reported moderate-to-severe signs of the disease over the previous week.43 Despite most participants receiving treatment, symptoms persisted across all treatment groups, indicating that the current management of CHE may be insufficient.43
The RWEAL (Real-World trEatment & mAnagement of chronic hand eczema in cLinical practice) study was a cross-sectional, retrospective chart review by physicians in Canada, France, Germany, Italy, Spain, and the UK.15 A total of 292 physicians completed forms for 1,939 patients with moderate-to-severe CHE who had been treated with TCS over the past 12 months.15
Results showed that treatment patterns, including the use of TCS, were generally similar in patients with moderate CHE and patients with severe CHE. More than one-in-four patients (27.4%) with moderate-to-severe CHE had progressed to phototherapy or systemic therapy. Among patients receiving topical treatment only, 17.2% were considered to have failed treatment with TCS (due to inadequate response or adverse events) or were contraindicated to TCS.15
These findings highlight the need for novel, effective, and well-tolerated treatment options specifically developed for patients with moderate-to-severe CHE in whom treatment with TCS is inadequate or inappropriate.15
Delgocitinib in Chronic Hand Eczema
Delgocitinib cream is a topical pan-JAK inhibitor that targets the activation of multiple JAK-STAT pathways involved in skin barrier dysfunction and the inflammation associated with CHE pathogenesis.44-46 It was recently approved in the EU for the treatment of moderate-to-severe CHE in adults for whom TCS are inadequate or inappropriate.46 A new drug application has also been accepted by the FDA.47
Safety and Efficacy of Delgocitinib in Chronic Hand Eczema
In the Phase III DELTA 1 and 2 trials of patients with moderate-to-severe CHE, delgocitinib cream 20 mg/g demonstrated significantly greater efficacy versus cream vehicle (each administered twice-daily),48 and was well tolerated when used as needed long-term.49,50 Sustained itch and pain reductions have also been reported in a Phase IIb trial.51
At FCDC, several posters presented data from a DELTA 1 and DELTA 2 pooled post hoc analysis of 639 patients treated with delgocitinib cream and 321 with a cream vehicle.52-54 Pooled data showed delgocitinib was clinically effective in clinician-reported outcomes (IGA-CHE treatment success and hand eczema severity index [HECSI]) and patient-reported outcomes (DLQI) over 16 weeks.50-52
Further analysis of these pooled data demonstrated that delgocitinib led to meaningful clinical improvements from Week 2 that continued to improve throughout the 16-week trial.52 Most delgocitinib-treated patients responded across the three disease domains of efficacy (≥90% improvement in HECSI score from baseline [HECSI-90]), symptoms (itch), and QoL (DLQI). At Week 16, clinically meaningful responses were achieved in at least one of the three disease domains by 80.1% of delgocitinib-treated patients (cream vehicle: 53.4%) and in all domains by 32.6% (cream vehicle: 10.4%).52 Most (82.0%) delgocitinib-treated patients achieved the clinically meaningful endpoint of IGA-CHE of 0/1/2.52
Another analysis used the HECSI score to investigate the impact of delgocitinib on signs of CHE and regions of the hand/wrist affected.53 More than two-thirds of delgocitinib-treated patients achieved clear/almost clear skin by Week 16 at least once.53 Statistically significant improvements with delgocitinib versus cream vehicle were seen across all signs of CHE and all regions of the hand and wrist up to Week 16.53
Lastly, the effect of delgocitinib on itch and pain were assessed in the pooled population.54 Reductions in itch and pain were significantly (p<0.001) greater in the delgocitinib group than the cream vehicle group at Day 1 (itch) and Day 3 (pain), and were maintained through to Week 16. 54 Clinically meaningful ≥4-point reductions in itch and pain were achieved by significantly more patients applying delgocitinib cream versus cream vehicle from Week 2 (p<0.001).54
Data were also presented from an analysis of systemic exposure and safety of delgocitinib in adults with moderate-to-severe CHE in the Phase III DELTA 2 study. Findings indicated that twice-daily application of delgocitinib cream 20 mg/g for 16 weeks resulted in minimal systemic exposure, and a favorable safety profile in the study population.55 The highest geometric mean plasma concentration of delgocitinib was 0.21 ng/mL at Week 1. AEs were reported by 45.7% (n=143/313) of patients in the delgocitinib cream group and 44.7% (n=71/159) of those in the cream vehicle group; COVID-19 was the most common AE. Few SAEs were reported, with none assessed as related to the study drug; no deaths were reported. Overall, data supported a lack of meaningful systemic effect from delgocitinib cream.54
The long-term safety and efficacy of delgocitinib cream for up to 36 weeks in adults with CHE was presented from the Phase III, open-label extension, DELTA 3 trial.56 In DELTA 3, delgocitinib cream 20 mg/g was administered as needed: patients with IGA-CHE 0 or 1 were not assigned to treatment, while patients with IGA-CHE ≥2 were assigned to delgocitinib. Consistent with DELTA 1 and 2, delgocitinib cream remained well tolerated in DELTA 3, and efficacy rates were maintained. Efficacy further improved among subjects previously treated with cream vehicle in a parent trial.56
Comparison of Delgocitinib with Systemic Therapies
Two posters were presented at FCDC 2024 demonstrating the favorable efficacy and safety of delgocitinib compared with systemic therapy.57,58
First, data were reported from the Phase III, multi-site, randomized DELTA FORCE trial that evaluated the efficacy and safety of delgocitinib cream compared to oral alitretinoin in adult patients with severe CHE.57 Note that alitretinoin is not approved for CHE in the USA. In DELTA FORCE, adults with severe CHE (IGA-CHE: 4) were randomized 1:1 to twice-daily topical delgocitinib cream 20 mg/g (n=254) or once-daily oral alitretinoin 30 mg (n=259) for up to 24 weeks.57
Superior treatment effects and QoL improvements were observed with delgocitinib cream 20 mg/g versus oral alitretinoin at Week 12, including significantly greater LS mean decreases in HECSI from baseline (p<0.001), and a greater proportion of patients achieving HECSI-90 (p=0.003) and IGA-CHE TS (p=0.004). Delgocitinib showed a favorable safety profile versus alitretinoin; fewer patients in the delgocitinib group reported AEs, serious AEs, and AEs leading to trial drug discontinuation.57
Second, data were reported from a matching-adjusted indirect comparison of the efficacy of delgocitinib and dupilumab in the treatment of moderate to severe atopic hand eczema.58 In the DELTA 1 and 2 trials, adult patients with moderate to severe CHE were randomized 2:1 to double-blind treatment with delgocitinib cream 20 mg/g or cream vehicle twice daily for 16 weeks, and in the LIBERTY-AD-HAFT trial, adults and adolescents with moderate-to-severe AD with H&F involvement were randomized to subcutaneous injection with dupilumab or placebo every 2 weeks for 16 weeks. Individual patient data from DELTA 1 and 2 were matched with aggregated baseline data from LIBERTY AD HAFT to provide an indirect comparison of these two treatments in the absence of head-to-head trials.58
The effective sample size after weighted matching was 201 patients from DELTA 1 and 2 (delgocitinib cream: n=128; cream vehicle: n=73). Results demonstrated that the efficacy of delgocitinib cream and dupilumab in patients with moderate-to-severe atopic hand eczema were comparable at 16 weeks. While differences were not statistically significant, all results were numerically in favor of delgocitinib cream.58
Conclusion
Overall, data presented at FCDC 2024 demonstrated that long-term use of tralokinumab is well-tolerated in patients aged 12 years and up with moderate-to-severe AD, including those ≥65 years.26,32 Tralokinumab treatment showed robust, stable, long-term efficacy with enduring control of disease activity,7,26,32 indicating that it is possible to transition from flare-driven treatment with topical therapies to stable disease control with long-term tralokinumab treatment.7 Data from real-world settings supported these findings, adding that tralokinumab offers an effective treatment option for managing moderate-to-severe AD regardless of prior dupilumab experience,31,59 and that it is efficacious in challenging regions of the body, including the H&N, H&F, and genitals.37-39
Delgocitinib cream was shown to be a well-tolerated topical treatment that led to improvements in CHE over both 16 weeks and 36 weeks,48,53,56 with associated improvements in itch, pain, and QoL.52,54 Delgocitinib demonstrated superior clinical treatment effects and QoL improvements, and a more favorable safety profile compared with oral alitretinoin,57 and the efficacy of delgocitinib was comparable with the biologic dupilumab in patients with moderate-to-severe atopic hand eczema.58 In addition, twice-daily application of delgocitinib cream 20 mg/g showed minimal systemic exposure.55 These data support the benefits of delgocitinib as an efficacious and well-tolerated topical treatment in this patient population which faces a high disease burden and has unmet treatment needs.57






