Meeting Summary
Ozanimod is an approved treatment for relapsing forms of multiple sclerosis (RMS) that has been shown to reduce relapses, new brain lesions, and brain volume loss relative to intramuscular interferon (IFN) β-1a.
This article summarizes selected data from clinical trials of ozanimod in RMS, which were presented at the 40th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) held in Denmark in September 2024 and the Annual Meeting of the European Charcot Foundation held in Italy in November 2024.
Final safety data were presented for the completed open-label extension (OLE) study of ozanimod in adults with RMS (DAYBREAK), showing that rates of adverse events (AE) remained stable or decreased over 8 years of ozanimod treatment. These findings confirm the established safety profile of ozanimod.
An analysis of brain volume changes across DAYBREAK and parent trials were also presented, demonstrating that the annualized rate of brain volume loss in participants treated with continuous ozanimod for up to 7 years was below the pathologic cutoff and was similar to those previously reported from healthy controls.
Data were presented from a retrospective, observational study into the effects of switching from fingolimod to ozanimod in patients with RMS due to safety or intolerance reasons. The authors concluded that this study confirms that switching from fingolimod to ozanimod for safety reasons such as lymphopenia or hypertransaminasemia may be a good strategy to continue sphingosine-1-phosphate receptor (S1PR)-modulating drug therapy.
Finally, an ad hoc interim analysis of patients with early RMS from ENLIGHTEN showed promising results, demonstrating that more than three-quarters of patients had either stable or improved cognitive processing speed after 1 year of ozanimod 0.92 mg.
Introduction
Ozanimod is a sphingosine 1-phosphate (S1P) receptor 1 and 5 (S1PR1 and S1PR5) modulator approved in multiple countries for the treatment of adults with RMS or moderately to severely active ulcerative colitis.1,2
In Phase III trials in patients with active RMS (RADIANCE and SUNBEAM), oral ozanimod 0.92 mg/day for up to 24 months was associated with a greater effect on clinically meaningful measures of disease activity, including relapses and brain MRI lesions, compared with IFN β-1a 30 μg/week.3,4 In addition, ozanimod significantly reduced the loss of whole brain volume (WBV), cortical grey matter volume (CGMV), and thalamic volume (TV) compared with IFN β-1a.3,4 Ozanimod was well tolerated, with fewer discontinuations due to treatment-related adverse events (TEAE) than IFN β-1a.3,4
Upon completion of either of the Phase III trials, all patients were eligible to enroll in the DAYBREAK OLE trial of ozanimod 0.92 mg, irrespective of parent trial study arm. This OLE study aimed to describe the long-term safety and efficacy patterns of ozanimod 0.92 mg in patients with RMS.5
The Phase IIIb ENLIGHTEN trial investigating the change in cognitive processing speed in patients with early RMS treated with ozanimod is also ongoing.6
Safety Patterns Over up to 8 Years with Ozanimod in Patients with Relapsing Multiple Sclerosis: Final Results from the DAYBREAK Open-label Extension Study
Krzysztof Selmaj, a neurologist at the University of Warmia and Mazury, Poland, presented a post-hoc analysis of the DAYBREAK OLE final safety results, including the 90.5% of patients who joined from Phase III parent trials (the ‘Phase III to DAYBREAK’ population). In addition, findings were presented from a further analysis limited to participants in this population who received continuous ozanimod 0.92 mg/day (the ‘continuous ozanimod 0.92 mg’ population).7 In both populations, rates of AEs remained stable or decreased over 8 years of ozanimod treatment.
At baseline in the Phase III parent trials, the mean (standard deviation [SD]) age of DAYBREAK OLE participants was 35.8 years (9.1), 66.6% were female, and most were White (99.4%) and Eastern European (90.9%). The mean (SD) time since multiple sclerosis (MS) symptom onset was 6.9 years (6.2), and the mean (SD) time since diagnosis was 3.7 years (4.5). Over a quarter (28.9%) of patients had received prior treatment with a disease-modifying therapy, and the mean Expanded Disability Status Scale (EDSS) score was 2.6. Patient demographics and characteristics were similar in the continuous ozanimod 0.92 mg population.
At the database lock for final data (April 7, 2023), the mean (SD) duration of ozanimod 0.92 mg exposure was 73.1 months (20.2) in the Phase III to DAYBREAK population (N=2,256), and 80.1 months (17.2) in the continuous ozanimod 0.92 mg population (n=762).
Overall, the proportion of patients treated with ozanimod 0.92 mg who experienced TEAEs in DAYBREAK decreased over time (Figure 1). The proportion of patients who experienced serious TEAEs remained stable over time. A total of 84 patients (3.4%) discontinued treatment during DAYBREAK due to AEs.
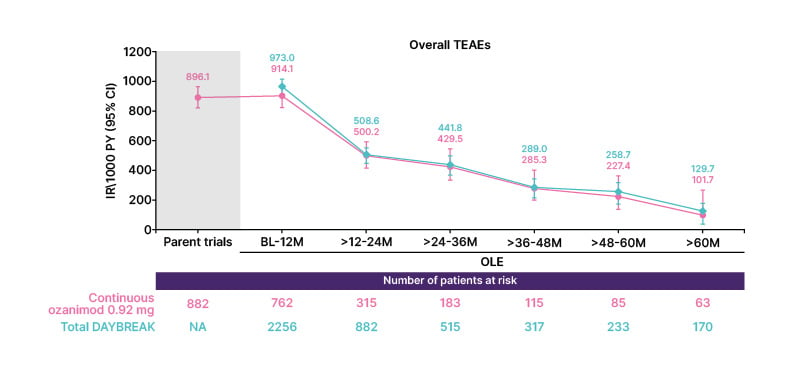
Figure 1: Proportion of patients treated with ozanimod 0.92 mg who experienced treatment-emergent adverse events during the open-label extension trial.
IR: incidence rate; M: months. OLE: open-label extension; PY: person-years; TEAE: treatment-emergent adverse event.
The incidence rates (IR) of infection TEAEs, serious infection TEAEs, and opportunistic infection TEAEs decreased or remained relatively low and stable over 60 months of ozanimod treatment. The most common infections occurring with continuous ozanimod 0.92 mg and in the overall OLE population included nasopharyngitis (161/762 and 483/2,256, respectively), COVID-19 (132/762 and 382/2,256, respectively), and upper respiratory tract infection (86/762 and 281/2,256, respectively). The most common serious infections were COVID-19 pneumonia (10/762 and 19/2,256, respectively), COVID-19 infection (7/762 and 19/2,256, respectively), and pneumonia (5/762 and 10/2,256, respectively). Oral herpes and herpes zoster were the most common opportunistic infections (continuous ozanimod 0.92 mg group, 12/762 each; overall OLE group, 50/2,256 and 38/2,256, respectively). Notably, herpes zoster infections did not increase in the overall OLE versus the parent trials. One serious opportunistic infection, a case of progressive multifocal leukencephalopathy, occurred in a patient who had received ozanimod 0.92 mg for approximately 4 years; the outcome was nonfatal with neurologic sequelae.
The IRs for malignancies, cardiac disorders, pulmonary disorders, and hepatic disorders also remained low in both the continuous ozanimod 0.92 mg group and the overall OLE population.8 The IRs for non-melanoma skin cancers, including basal cell carcinomas, decreased from the parent trials through the OLE. During DAYBREAK, the IR of non-melanoma skin cancer was 102.9 per 100,000 person-years, compared with an estimated age-standardized IR in North American and European populations of between 22.1–795.7 per 100,000 people in 2019.9 Elevations of ≥3 times the upper limit of normal of alanine aminotransferase, aspartate aminotransferase, and bilirubin were rare. There were a few confirmed macular edema cases: 1/792 in the continuous ozanimod 0.92 mg group and 5/2,256 cases in the overall OLE population.
Selmaj K et al.7 concluded that these post hoc data demonstrate that the rate of TEAEs generally declined or remained stable over time in patients with RMS treated with ozanimod 0.92 mg/day for up to 8 years. These findings therefore confirm the established safety profile of ozanimod in patients with RMS.
Whole Brain, Cortical Grey Matter, and Thalamic Volume Changes During 5–7 Years of Ozanimod in Relapsing Multiple Sclerosis: Final Results from the DAYBREAK Open-Label Extension Study
Jeffrey Cohen, Director of Experimental Neurotherapeutics at the Cleveland Clinic Mellen Center for Multiple Sclerosis Treatment and Research in Ohio, USA, presented final data from the DAYBREAK OLE, showing that annualized rates of brain volume loss in participants treated with continuous 0.92 mg/day for 7 years are similar to those previously reported from healthy controls.10
The normal process of aging is associated with a small amount of brain volume loss of <0.4% per year.11 However, people with MS may experience an accelerated rate of brain volume loss (>0.4% per year), which is associated with impaired cognitive function, physical disability progression, and decreased quality of life.11-14
Cohen J et al.10 presented data from an analysis of final DAYBREAK OLE data that aimed to evaluate rates of brain volume loss in participants treated with ozanimod for 5–7 years. In this analysis, demographics and baseline disease characteristics at the parent trial baseline were generally similar across studies and between each treatment group. The mean age of participants was 35–36 years (depending on the group), 63–70% were female, and the majority were White (>99%). Time since MS symptom onset varied between 6–7 years, and time since MS diagnosis between 3–4 years, with a mean number of relapses in the 12 months prior to the parent trial of 1.3 in all groups.
Participants receiving continuous ozanimod 0.92 mg had stable, low rates of WBV loss through to DAYBREAK Month 60 (annualized last squares mean percentage [LSM%] change from parent baseline: RADIANCE, −0.27; SUNBEAM, −0.35; Figure 2). In participants treated with continuous ozanimod 0.92 mg for the longest duration (2 years in RADIANCE plus 5 years in DAYBREAK), the rate of brain loss (LSM% [standard error]: WBV −0.27 [0.02], CGMV −0.30 [0.02], and TV −0.57 [0.04]) were similar to those previously reported from healthy controls (mean [SD]: WBV −0.23 [0.12], CGMV −0.28 [0.32], and TV −0.48 [0.10]).15
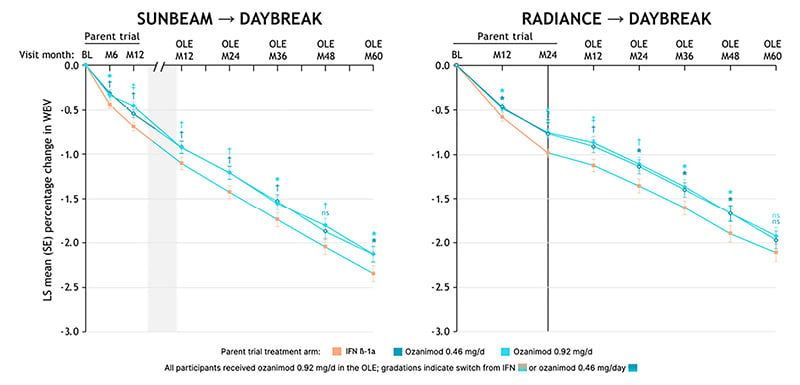
Figure 2: Annualized whole brain volume loss relative to RADIANCE baseline.
BL: baseline; d: day; LS: least squares; M: month; OLE: open-label extension; WBV: whole brain volume.
Compared with participants who switched from IFN β-1a, those receiving continuous ozanimod 0.92 mg had statistically significantly lower LSM% reductions in WBV from RADIANCE baseline through to DAYBREAK Month 48, and from SUNBEAM baseline through to DAYBREAK Month 60 (each nominal p<0.05; Figure 2).
In participants from RADIANCE, switching from IFN β-1a to ozanimod reduced rates of WBV loss (Figure 2). For example, the annualized LSM% change from baseline to Month 24 in RADIANCE was −0.48, whereas the annualized LSM% change from baseline to Month 24 in DAYBREAK was −0.19. A similar pattern was observed in participants from SUNBEAM, and for annualized LSM% change in TV.
Participants treated with IFN β-1a experienced high annualized reductions in CGMV from baseline (−1.02 at Month 12 in SUNBEAM and −0.59 at Month 24 in RADIANCE). However, this trend reversed 12 months after switching to ozanimod in DAYBREAK, with an annualized LSM% increase relative to DAYBREAK baseline of 0.10 (SUNBEAM participants) or 0.20 (RADIANCE participants; Figure 2).
Cohen J et al.10 concluded that rates of brain volume loss in ozanimod-treated participants with RMS decreased during the randomized parent studies compared with baseline, and these reduced rates were sustained with continuous ozanimod 0.92 mg/day treatment for up to 5 years in DAYBREAK. He stressed that the annualized rates of WBV loss in participants treated with continuous ozanimod 0.92 mg for the longest duration (2 years in RADIANCE plus 5 years in the DAYBREAK OLE) was below the pathologic cutoff of 0.4% and was similar to that previously reported from healthy controls.15 In addition, switching from IFN β-1a to ozanimod consistently reduced rates of brain volume loss.
Switch from Fingolimod to Ozanimod for Safety or Intolerance Reasons
Elisabetta Signoriello, neurologist at the University of Campania Luigi Vanvitelli in Naples, Italy, presented data from a multicenter, retrospective, observational study showing that switching patients from fingolimod to ozanimod can mitigate the incidence of lymphopenia and hypertransaminasemia.16
Fingolimod and ozanimod are both S1PR-modulating drugs approved for the treatment of patients with RMS.1,2,17,18 While fingolimod binds non-selectively to S1PR1, S1PR3, S1PR4, and S1PR5, with well-known cardiovascular, ophthalmologic, pulmonary, and hepatic safety concerns,17,18 ozanimod is a relatively new molecule that selectively targets S1PR1 and S1PR5,1,2,17 thereby minimizing the potential safety concerns around S1PR3 activation.17-20
Indirect comparative studies based on clinical trials indicate that ozanimod may have an improved safety profile compared with fingolimod, with a lower risk of any AEs, including bradycardia, lymphopenia, and elevated liver enzymes.19
The objective of this retrospective study was to compare the efficacy, safety, and persistence of treatment in patients with MS who switched from fingolimod to ozanimod for safety reasons. A total of 60 patients were enrolled, of whom 61.6% were female. EDSS at baseline was 2.4, and the mean duration of fingolimod treatment prior to switching was 5.7 years (SD: 3.3). Of note, 28.5% of patients were treated with an off-label dose of fingolimod; two patients skipped their dose on Sundays, while the remainder received a dose every other day (daily dosing is the approved schedule).17,18 The majority of patients switched treatment from fingolimod due to lymphopenia (70%), with 22% switching for hypertransaminasemia and 7% for chronic inflammatory bowel disease. After switching to ozanimod, patients were followed up for a mean of 1.5 years (SD: 0.49).
The mean time between discontinuation of fingolimod and initiation of ozanimod (the washout period) was 23.3 days (range: 1–180 days). Among patients who switched due to lymphopenia, the mean washout period was 24.3 days (SD: 23.4), while among those who switched due to hypertransaminasemia, this period was a little longer, at 27.1 days (SD: 25.2).
In terms of efficacy, there was a statistically significant reduction in annualized relapse rate from the year before to the year after switching to ozanimod (0.26 [SD: 0.44] and 0.02 [SD: 0.15], respectively; p=0.004; Table 1). However, the authors noted that this improvement may be partly due to the reduced dosage of fingolimod that some patients were receiving prior to the switch. The majority of patients (97%) persisted with ozanimod treatment beyond the 1.5 years of study follow-up. One patient discontinued due to clinical relapse, and one for planned pregnancy.
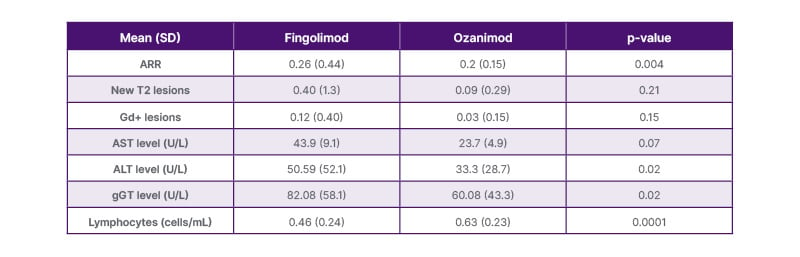
Table 1: Comparative efficacy and safety between fingolimod and ozanimod treatment in patients who switched to ozanimod.
ALT: alanine transaminase; ARR: annualized relapse rate; AST: aspartate transaminase; gd+: gadolinium enhancing; gGT: gamma glutamyl transpeptidase; SD: standard deviation; T2: detected through the T2 magnetic resonance imaging method.
Mean lymphocyte count increased statistically significantly from measurements taken during fingolimod treatment to those taken during ozanimod treatment (0.46 to 0.63 cells/mL, respectively; p=0.0001; Table 1). Mean lymphocyte count also increased among lymphopenic patients, from 0.39 to 0.56 cells/mL (p=0.025). No patients discontinued ozanimod due to lymphopenia during the follow-up period; however, one patient did develop Grade 3 lymphopenia during ozanimod treatment.
A reduction in the number of patients with hypertransaminasemia was observed following the switch to ozanimod (from 20.9% to 9.3%; p=0.03). This trend was seen across aspartate aminotransferase, alanine aminotransferase, and gamma glutamyl transpeptidase measures in both the overall study population (Table 1) and in those patients who switched due to hypertransaminasemia.
Results were also analyzed in terms of patients with no adverse drug events (NADE) and those with no evidence of disease activity or progression (NEDA-3). After the switch to ozanimod, 93% of patients in the study were NADE during the follow-up period, and 88.3% were NEDA-3 after 1 year on ozanimod. The percentage of patients with both NADE and NEDA-3 after switching was 83.7%.
These data indicate that patients with lymphopenia who switch from fingolimod to ozanimod adjust to the change well, and lymphopenia is improved. Similar improvements in liver enzymes were shown in patients who switched to ozanimod due to hypertransaminasemia. The authors concluded that this study confirms that switching from fingolimod to ozanimod for safety reasons such as lymphopenia or hypertransaminasemia may be a good strategy to continue S1PR-modulating drug therapy.
Symbol Digit Modalities Test and Safety Outcomes at 1 Year in the ENLIGHTEN Study of Ozanimod in Early Relapsing Multiple Sclerosis
Robert Naismith, a neurologist at the Washington University School of Medicine, USA, presented interim data from the ongoing 3-year, single-arm, multicenter, prospective Phase IIIb ENLIGHTEN study of ozanimod in patients with early RMS. Following screening, patients began a 7-day dose-titration to the final dose of 0.92 mg/day for 36 months.The primary endpoint in ENLIGHTEN is the proportion of patients with an increase in cognitive processing speed from baseline to Year 1, as measured by an improvement in Symbol Digit Modalities Test (SDMT) score of ≥4 points, or 10%. The SDMT was administered orally in ENLIGHTEN.
A total of 188 patients were enrolled and received ≥1 dose of ozanimod. The mean (SD) age at baseline was 39.5 (10.7) years, and 78.7% of patients were female. The mean (SD) time since MS symptom onset was 4.1 (5.5) years, and the mean (SD) time since MS diagnosis was 1.0 (1.3) years, representing a population with early RMS. The mean (SD) baseline SDMT score was 53.4 (12.0). Most patients (70.7%) were naïve to disease-modifying therapy (DMT). The median EDSS score was 2 (range 0–4) and the mean (SD) SDMT score was 53.4 (12.0). The mean (SD) number of relapses in the prior 12 months was 0.8 (0.8) (34.0% were relapse-free). Patients had a mean (SD) of 0.8% (1.6%) gadolinium-enhancing lesions (66.5% were gadolinium-enhancing lesion free), and a mean (SD) of 22.3 (16.8) T2 lesions.
At the time of data cutoff, February 7, 2024, 15 of the 188 enrolled patients had completed treatment, and 119 were on treatment.
The mean (SD) ozanimod exposure among all enrolled patients at this point was 22.0 (9.4 months), or 344.1 person-years. Among those patients with SDMT data at 1 year (n=168), 48.2% had an improvement in SDMT score, 30.4% had stable SDMT, and 21.4% had worsened. When these data were adjusted for baseline SDMT value, baseline age, baseline WBV, and time point, an estimated 48.1% of patients experienced an improvement in SDMT.
In terms of safety parameters, 81.4% of the overall population had experienced ≥1 TEAE during the study by the time of data cutoff. The most common TEAE was COVID-19 (25% of patients), which was expected since the study began just before the start of the pandemic, followed by headache (11.7%), urinary tract infection (10.6%), and fatigue (10.1%). Rates of serious TEAEs (6.9% of patients) and TEAEs leading to permanent ozanimod discontinuation (3.2%) were low.
Naismith et al.21 concluded that more than three-quarters of patients with early RMS experienced stable or improved cognitive processing speed during 1 year of ozanimod, and that TEAEs (other than COVID-19) were largely consistent with those reported in the overall ozanimod clinical development program.







