Abstract
Obesity is a common overlapping risk factor for cancer and cardiovascular disease (CVD), and the long-term consequences of these chronic, interconnected diseases are severe. The importance of CVD in breast cancer (BC) patients and survivors has been well-established, and the potential impact of some BC treatments (such as cardiotoxic effects related to chemotherapy or targeted therapy with the use of doxorubicin or trastuzumab, and radiation therapy, especially in cases of left breast tumours) on the cardiovascular condition necessitates ongoing cardiological surveillance. In addition, the possible reduction of some underlying risk factors is critical to long-term protection of BC patients and survivors.
The concept of obesity dynamically interacting with both BC and CVD is important because it is a modifiable risk factor, and the modern management of obesity deserves emphasis. In particular, for many BC patients and survivors, an effective weight reduction programme integrated with standard anticancer and cardiology therapies can improve patient outcomes.
This review presents the complex relationships between overweight, obesity, CVD, and BC risk and highlights outcomes in post and premenopausal women, focussing on patients with hormone receptor-positive BC. The review provides evidence from epidemiologic, observational, and weight loss intervention trials which have examined the effects of weight reduction programmes on BC outcomes. Such studies have indicated that moderate weight loss, with regular physical exercises or stress reduction, can significantly improve BC outcomes. Future lifestyle intervention trials could support the incorporation of weight loss interventions as an integral element of comprehensive management for BC patients and survivors.
INTRODUCTION
There is a connection between increased body mass, or adipose tissue, and higher risk of developing cancer, particularly in hormone-stimulated neoplastic diseases, such as breast cancer (BC), especially among postmenopausal women.1,2 In addition, obesity and BC are interrelated via complex hormonal and molecular signalling networks.3 Obesity is also an important risk factor for cardiometabolic diseases, including coronary heart disease, arterial hypertension, Type 2 diabetes mellitus (T2DM), and dyslipidaemia.4 Moreover, the toxicity of some anticancer therapies can adversely impact both oncologic and cardiac outcomes. This can be especially dangerous in women with HER2-positive and hormone receptor (HR)-positive biological subtypes of BC.4
At present, numerous patients with malignancies are living longer than ever before because of early detection and the progression of modern oncology therapies.4 In addition, several new therapies for cardiovascular disease (CVD) are being developed.4 In this situation, patients who are receiving anticancer treatments and cancer survivors are at risk of subclinical toxicity of different medications, including the negative impact anticancer therapies can have on the cardiovascular (CV) system.4 Achieving a proper balance between antineoplastic treatment and CV safety is essential in daily practice. While the main efforts of medical practitioners are appropriately targeted on the treatment of BC and CVD, obesity, which is often linked with both these conditions, remains unaddressed. In response to this challenge, this article details recent research and strategies to motivate various members of medical teams to work closely with their BC patients and survivors who are obese. Since obesity is a modifiable risk factor for BC and CVD, accomplishing a moderate, but sustained, weight reduction is a big step forward in terms of improving long-term outcomes for both BC and CVD.
OBESITY AND ITS CONNECTIONS WITH MALIGNANCIES
An imbalance in the endocrine regulation of adipose tissue metabolism often leads to an abnormal fat accumulation and an excess of body mass, causing obesity. Commonly used anthropometric markers of obesity include BMI, waist circumference, and waist-to-hip ratio (measured by waist circumference divided by hip circumference).5 Individuals can be divided into classes based on their BMI: underweight (<18.5), normal weight (18.5–24.9), overweight (25.0–29.9), or obese (≥30.0), and the waist-to-hip ratio reflects the distribution of the body adiposity by indicating whether more fat is located in the abdomen or the hips.6 Current research indicates that higher body adiposity is linked to an elevated risk of several cancers, including breast, endometrial, ovarian, oesophageal, gastric, colorectal, liver, renal, pancreatic, thyroid, gallbladder, multiple myeloma, and meningioma.1 Several studies have shown that an elevated BMI was related to increased risk of BC, especially in postmenopausal women. For instance, obese postmenopausal women had a 20–40% increased risk of developing HR-positive BC, compared with normal weight women.2 In contrast, overweight and obese premenopausal women had a 20% decreased risk of HR-positive BC compared to normal weight premenopausal women.2
COMMON MECHANISMS BY WHICH OBESITY MAY INCREASE THE NEOPLASTIC RISK
Obesity can increase the risk of cancer via several mechanisms that are addressed below. Obese individuals can have chronic, low-level inflammation that can cause damage to DNA, leading to neoplastic lesions.7 Furthermore, adipose tissue produces an excessive amount of oestrogen that has been related to an elevated risk of hormone-stimulated cancers, such as breast, endometrial, ovarian, and various other malignancies. In addition, obese individuals may be affected by hyperinsulinaemia, insulin resistance, or pre-diabetes, which are disorders that can precede the development of T2DM. Obese and overweight patients often have high blood levels of insulin and insulin-like growth factor-1, which may promote the development of breast, endometrium, colon, kidney, or prostate malignancies.8 Adipose cells produce adipokines, tissue hormones such as leptin and adiponectin, that can stimulate or inhibit cellular growth or proliferation. Particularly in obese individuals, the level of leptin in the blood increases with growing body mass and fat, and there is a lower level of adiponectin, which has antiproliferative effects, compared to people with a normal body weight. Moreover, adipose cells can have direct or indirect effects on some cell growth regulators, such as a mTOR or adenosine monophosphate (AMP)-activated protein kinase. Examples of additional biological mechanisms that connect obesity and cancer risk include changes in the immune response, alterations in the NFκB system, and a higher level of oxidative stress.9
THE CONNECTIONS BETWEEN BREAST CANCER, OVERWEIGHT, AND OBESITY
The relationship between obesity and BC is complex. BMI has been established as a risk factor for BC, which influences treatment outcomes, predominantly in postmenopausal women.10 In particular, it has been shown that overweight and obese women with BC have an elevated risk of distant metastases compared with patients who have a BMI within the normal range.11 Mechanisms connecting obesity and BC include the endocrine and metabolic effects of excessive body mass and fat content, as well as the changes that they cause in molecular signalling pathways and endocrine communication.3 A meta-analysis has found that each 5 kg increase in adult weight gain was correlated with an 11% increased risk of postmenopausal BC among women who were not using hormone replacement therapy. However, there was no similar relationship between obesity and BC in the case of premenopausal BC.12 Results of the Nurses’ Health Study and Nurses’ Health Study II trials have shown that excessive body mass and adiposity in childhood and adolescence were correlated with a 20–50% decrease in the risk of BC across the lifespan, regardless of menopausal status. However, a positive connection has been noted between a short-term weight gain, pre and postmenopausal invasive BC, and HR status, after adjusting for pre and postmenopausal BMI.13 This correlation was stronger for premenopausal compared with postmenopausal women. Increased risk of BC among postmenopausal women who were lean in adolescence was found in those who had excessive weight gain during adulthood.14 In addition, in BC survivors, obesity can have an adverse influence on cancer recurrence, complications, and quality of life.15
THE RELATIONSHIP BETWEEN OBESITY AND CARDIOVASCULAR DISEASE
Overweight and obesity represent risk factors for cardiometabolic diseases, such as T2DM, arterial hypertension, and dyslipidaemia. Furthermore, the adverse metabolic effects of excess body adiposity accelerate the progression of atheromatic lesions that can, in turn, lead to atherogenic CVD, such as coronary heart disease, stroke, or premature cardiac death.4 Overweight and obesity, represented by a BMI of ≥25 and ≥30 kg/m2, respectively, are important modifiable risk factors for CVD.16 Moreover, excess body mass and central adiposity, which are often associated with physical inactivity, can augment the risk of CVD. In particular, class III obesity (BMI ≥40 kg/m2) can present a higher CV risk than class I (BMI 30–35 kg/m2) or class II (BMI 35–40 kg/m2) obesity among post menopausal women, which is the case across different ethnic populations.17 Furthermore, there is a link between obesity and thrombosis, including increased expression of the prothrombotic plasminogen activator inhibitor-1 and tissue factor, as well as elevated activity of platelets. Additionally, risk factors for venous thromboembolism among patients with obesity include inflammation, increased thrombin production, decreased fibrinolysis, and platelet hyperactivity.18 Proinflammatory and prothrombotic factors can aggravate both atherogenic CVD and malignant lesions.
OBESITY: NEW INSIGHTS INTO THE CONTROL OF ENERGY BALANCE
A wide spectrum of genetic, metabolic, behavioural, cultural, and environmental elements contribute to obesity, which has significant medical, psychological, social, and economic consequences.19 A real breakthrough in obesity research occurred following the discovery that the central nervous system is involved in the accumulation of body fat. The brain plays a key role in the coordination of appetite and body mass.20 The hypothalamus is the main brain area responsible for the central control of energy intake and expenditure, and the arcuate nucleus in the hypothalamus regulates feeding behaviour and several metabolic pathways.21 Such a system provides flexible responses to challenges related to controlling the energy balance (Table 1).22-24 In these circumstances, sensory, emotional, and psychosocial factors, such as stress, can adversely affect feeding behaviour and body weight, mostly via non-homeostatic mechanisms.25 On the one hand, the abnormal accumulation of fat tissue can be explained in terms of a disequilibrium between energy intake and expenditure. On the other hand, the mechanisms that determine energy intake and expenditure, in the current environment, are dominated by overwhelming obesogenic factors, such as aggressive food advertisement and the availability of nutrient-depleted, highly processed food, that are beyond simple control.26,27
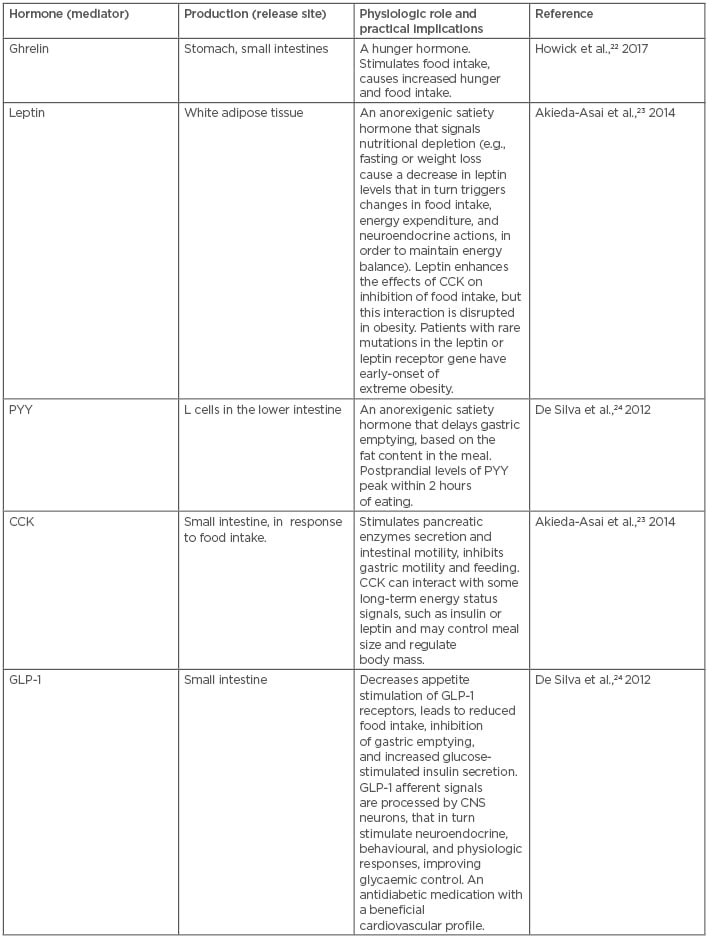
Table 1: Main signals involved in the homeostatic regulation of appetite and satiety.
CCK: cholecystokinin; CNS: central nervous system; GLP-1: glucagon-like peptide-1; PYY: peptide YY.
IMPORTANT BENEFITS OF MODERATE WEIGHT LOSS: SMALL STEPS TO IMPROVE LONG-TERM OUTCOMES
Lifestyle modifications in diet, behaviour, and physical activity have been recommended as a primary step in weight reduction.28
Typically, weight loss occurs over 4–6 months, during which time the patients lose 4–10% of their initial body mass. Such a weight reduction is usually followed by a plateau that is followed by weight regain.29 Since long-term weight loss is still difficult to achieve for many patients, the addition of pharmacotherapy can be considered.30 The main goal of pharmacotherapy for obesity is to inhibit the biological patterns of weight gain and attenuate the counter-regulatory response of the organism to weight reduction. Improved control of these mechanisms should allow patients to lose weight and sustain body mass reduction.31 A moderate and sustained body mass reduction, of 5–10% for example, is crucial to improve CV, metabolic, and other disease outcomes.32 Additionally, weight reduction can decrease proinflammatory markers, such as C-reactive protein, TNF-α, and IL-6.33 Moreover, weight loss leads to the decrease of insulin secretion and insulin resistance. This, in turn, lowers the risk of CVD and several malignancies.34 Weight loss can also decrease oestrogen levels, contributing to the reduction of HR-positive BC risk.35
INTEGRATION OF WEIGHT REDUCTION AND PHYSICAL ACTIVITY IN BREAST CANCER PATIENTS AND SURVIVORS
The transition from an active anticancer treatment into survivorship is known as the re-entry phase. This can take place during the first year, after completing the adjuvant treatment regimen. The re-entry phase is a convenient stage of the cancer continuum that can be used for education and long-lasting behavioural changes. At that time, the management priorities shift from diagnosis and treatment to long-term survivorship.36 In fact, the re-entry phase is a prime opportunity for medical teams to actively promote the advantages of healthy behaviours. Studies have shown that losing weight reduces BC risk. In a recent meta-analysis of data from >4 million individuals, weight loss was associated with an 18% relative reduction in BC risk. In addition, exercise, which is often connected with a weight reduction programme, contributed to a 22% relative risk reduction of BC.37 Furthermore, physical activity may help to decrease some adverse effects of anticancer therapies. For instance, 4 months of resistance and high-intensity training reduced the cancer-related fatigue and symptom burden in BC patients receiving chemotherapy (CHT).38 Performing exercises 1 day before a scheduled CHT, such as doxorubicin, improved the patient’s mood and reduced the musculoskeletal side effects of the CHT.39
THE INVERSE CORRELATION OF PREMENOPAUSAL HORMONE RECEPTOR-POSITIVE BREAST CANCER WITH OVERWEIGHT AND OBESITY: A CHALLENGING TOPIC FOR FURTHER STUDIES
BC has a complex aetiology, in which the body adiposity, commonly assessed via BMI, seems to have opposing effects in postmenopausal and premenopausal patient populations. For example, BC risk is lower premenopause and increases after menopause.40 Research evidence has shown that the elevated postmenopausal adiposity is positively correlated with BC risk, which is partly due to the increased oestrogen production by excess adipose cells, such as the contribution to HR-positive BC development in the postmenopausal population.40-42 In addition, increased fat tissue often triggers chronic, low-grade inflammation, which is correlated with a higher risk of BC recurrence.40 However, according to a recent study, premenopausal women with a higher BMI may have a lower HR-positive BC risk.43 The results of this study need to be interpreted with caution, due to its limitations.43 Firstly, this was an observational study; therefore, the findings are not as strong as with a randomised controlled trial (RCT). Secondly, the study used BMI as a measure of the body adiposity, which is not accurate since participants with an identical BMI may have a different amount and distribution of body fat. Thirdly, the weight and height measurements were self-reported, and therefore may have been inaccurate, which would have influenced the findings. Nevertheless, the results of this large, international analysis of data on premenopausal women, aged 18–54 years, have suggested that increased adiposity is associated with a decreased risk of premenopausal BC to a greater degree than previously revealed.43 In addition, the strongest association of BC risk was found for elevated BMI in early adulthood, at 18–24 years old.43 At first glance, these findings may seem promising for overweight or obese premenopausal women, in whom an increased adiposity appears to be a protective factor.43 However, it should be noted that since obesity has several negative effects on health, weight gain should certainly not be recommended for the prevention of BC among premenopausal women.43 In addition, further studies explaining the exact biological reasons behind the inverse correlation of premenopausal fat with HR-positive BC risk might detect factors contributing to BC, which may have important implications for clinical practice.43
EPIDEMIOLOGIC, OBSERVATIONAL, AND WEIGHT LOSS INTERVENTION TRAILS EXPLORING THE EFFECTS ON BREAST CANCER OUTCOMES
Many epidemiologic and observational studies have revealed that obesity, weight gain, and a low level of physical activity have been associated with worse outcomes and survival among women with BC.40,44 For instance, in a large study, the impact of prognostic factors on BC-specific mortality and non-BC-related mortality, among women with an early stage BC registered in the Surveillance, Epidemiology, and End Results (SEER) programme, was investigated.45 In general, these women had a good oncologic prognosis. Since most of these patients had died from non-BC-related causes, which are often lifestyle-related, a health-oriented lifestyle together with the comprehensive management of comorbidities should be a priority in such patients.45 Most women with BC are either overweight or obese at diagnosis, and while they are coping with BC treatments, they are usually unable to lose weight on their own and would benefit from implementing standardised nutrition and exercise programmes.46 This is in agreement with a recent systematic review that suggested that weight loss is feasible and safe among women who underwent treatment for BC.47 Similarly, the prospective randomised WINS trial examined the effects of a low-fat diet intervention and demonstrated a mean 3.7% weight loss and decrease in the risk of BC recurrence.48 Other RCT that were designed to determine whether diet and exercise can improve BC outcomes include the Canadian LISA study49 and the ENERGY trial,50 which have shown the benefit of weight loss in BC patients and survivors. Recently, the large-scale, ongoing BWEL RCT has been conducted by the Alliance of Clinical Trials in Oncology. The BWEL trial will examine overweight or obese, premenopausal and postmenopausal patients with BC participating in health education programmes, with or without weight loss intervention, such as loss of 10% of baseline body mass achieved via caloric restriction and increased physical activity, within 1 year of BC diagnosis.51 The BWEL trial will explore the impact of weight loss on the risk of BC recurrence and mortality.51
It is expected that the results of the BWEL trial, which should be available in 2023, will provide convincing data regarding the introduction of a weight loss programme as a standard component of BC management.51
THE WEIGHT MANAGEMENT PROGRAMME: JOINT EFFORTS OF MEDICAL TEAM MEMBERS AND PATIENTS
Physicians often have insufficient time to spend during visits with their patients, and thus a close collaboration with dietitians, pharmacists, and nurses is necessary. These practitioners may often devote much more time to the patients than busy physicians. Moreover, some baseline strategies for coping with chronic stressors aimed at improvement of response to stress, and better control of symptoms, should be adopted by BC patients and survivors (Table 2).36 Providing such integrated services, together with the educational and behavioural interventions, such as addressing common obstacles to effective co-operation with the medical teams (Table 3),37,52,53 will improve the psychophysical condition, quality of life, and resilience of many patients with BC and CVD.
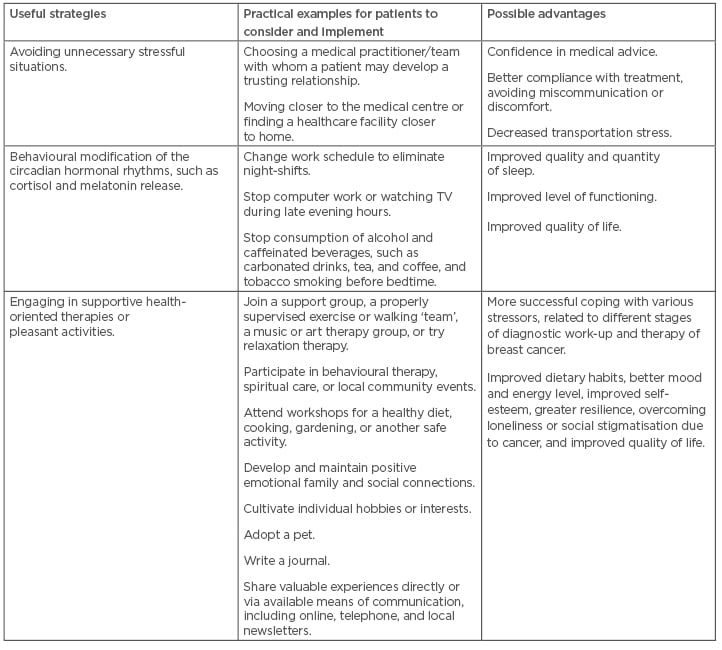
Table 2: Strategies to improve coping with chronic stress in breast cancer patients and survivors.36
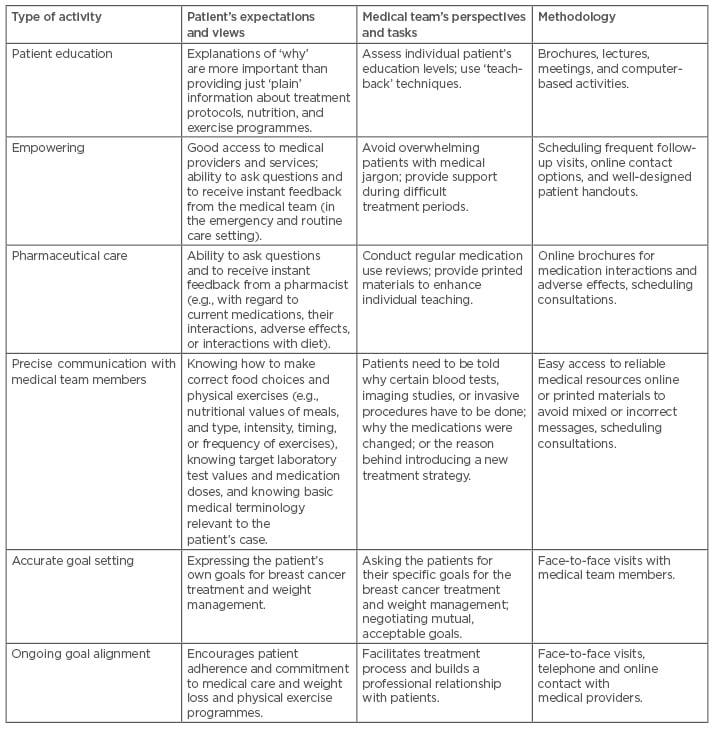
Table 3: Possible activities to enhance self-management among obese breast cancer patients and survivors.37,52,53
CONCLUSION
In summary, BC outcomes are dependent, to a certain degree, on the concurrent CV status and BMI or adipose mass, before, during, and after completing the BC therapy cycle. In addition, the combined efforts of oncology and cardiology specialists, and primary care providers, together with the efficient co-operation with well-educated patients, are crucial to improving BC outcomes and reducing adverse influences of anticancer therapies on the CV condition. In the advent of personalised oncology that applies different targeted therapies to patients with BC, there is a large population of women with excess body weight at risk of possible CV complications. A rapidly growing population of BC survivors, with cardiometabolic comorbidities, represents a big challenge. Under these circumstances, it is imperative to prevent current BC patients from becoming future cardiac patients. In fact, such patients should be the most motivated group since a sustained weight reduction can save their lives. It is expected that further studies will determine whether sustained weight loss can lead to better outcomes among patients with BC. Hopefully, future prospective, large-scale, randomised trials, combining lifestyle interventions with standard medical treatment will support the systematic incorporation of structured weight loss interventions into routine management of patients with BC.








