Abstract
In patients with breast cancer, the expression of oestrogen receptor, progesterone receptor, and human epidermal growth factor 2 (HER2) is used as a molecular marker to determine prognosis and direct treatment decisions; however, this does not fully reflect the molecular complexity of the disease. Patients with early-stage hormone receptor-positive (ER+), HER2-negative (HER2-) breast cancer are typically treated with surgery, followed by adjuvant systemic endocrine therapy with or without adjuvant radiation therapy. Gene expression profiling assays complement clinicopathological parameters, such as tumour size, grade, and nodal status, and can be used to classify risk of recurrence, thereby informing adjuvant therapy decision-making in early-stage breast cancer to prevent unnecessary treatment with chemotherapy in low risk patients.
In this review, the authors evaluate the evidence to date supporting the use of one of the tests, the Prosigna PAM50 risk of recurrence assay (Nanostring, Seattle, Washington, USA), as a prognostic tool in ER+/HER2- early-stage breast cancer, and summarise findings from a clinical and cost-effectiveness analysis performed by the National Institute for Health and Care Excellence (NICE) in the UK. The authors also focus on recommendations from regulatory bodies and key ongoing research efforts to address the remaining uncertainty regarding the application of available genomic signatures in ER+/HER2- early-stage breast cancer.
INTRODUCTION
Over 70% of patients with early-stage breast cancer are diagnosed with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) disease.1 These patients are typically treated with surgery (mastectomy or breast conserving), followed by adjuvant systemic endocrine therapy with or without adjuvant radiation therapy.2,3 As per European guidelines, the majority of HR+/HER2-/luminal A-like tumours are treated with endocrine therapy alone, although chemotherapy can also be included in high-risk cases.2 For HR+/HER2-/luminal B-like tumours, indications for chemotherapy depend on endocrine receptor (ER) expression (oestrogen/progesterone), proliferation (Ki67 expression), genomically assessed risk, tumour burden, and/or patient preference.2
Clinicopathological parameters, including tumour size, grade, nodal status, HR status, HER2 status, and Ki67 status, are commonly used as part of staging or risk prediction algorithms to estimate the risk of breast cancer recurrence and aid decision making for adjuvant treatment.2-4 However, in early-stage breast cancer the benefit of chemotherapy does not appear to vary based on clinicopathological factors alone.5
While the expression of ER, progesterone receptor, and HER2 is used as a molecular marker to stratify patients with specific breast cancer subtypes for treatment and prognosis,6,7 this does not fully reflect the molecular complexity of the disease. The addition of information from multi-gene profiling has the potential to improve the accuracy of prognosis, and a benefit of improved risk stratification is the ability to identify patients with sufficiently good prognosis (low risk of recurrence [ROR]), for whom the addition of cytotoxic chemotherapy is not warranted. Gene expression profiling (GEP) assays complement clinicopathological parameters, such as tumour size, grade, and nodal status, by quantifying multiple genes and combining into multivariate prediction models. Multigene prognostic tests have been available for over a decade, with EndoPredict (Myriad Genetics, Munich, Germany), MammaPrint (Agendia, Amsterdam, Netherlands), Oncotype DX Breast Recurrence Score (Genomic Health, Redwood, California, USA), and the Prosigna PAM50 ROR assay (NanoString Technologies, Seattle, Washington, USA) being developed to classify the ROR and inform adjuvant therapy decision making in early-stage breast cancer.7 Non-commercial assays include the Clinical Treatment Score (CTS) and 4-marker immunohistochemical score (IHC4); however, these assays are based on immunohistochemical/clinicopathological information only, without a molecular component, and are therefore not a focus of this review.8
Here the authors evaluate the evidence to date for the use of Prosigna as a prognostic tool in ER+/HER2- early-stage breast cancer and provide an overview of a recent clinical and cost-effectiveness analysis approach used by the National Institute for Health and Care Excellence (NICE). The authors also summarise recommendations from regulatory bodies and key ongoing research efforts to fulfil remaining clinical gaps/uncertainty regarding the applications of available genomic signatures.
COMMERCIALLY AVAILABLE GENOMIC ASSAYS IN EARLY-STAGE BREAST CANCER
First-generation assays developed for use in ER+/HER2- breast cancer included MammaPrint and the Oncotype DX Breast Recurrence Score, both of which are prognostic in early-stage breast cancer9,10 but can only be performed in central laboratories and do not include clinicopathological factors. Further technological and scientific advances led to the development of second-generation prognostic assays, including EndoPredict and Prosigna, which have now been validated and can be performed in local laboratories,11,12 potentially reducing the overall cost of the assay and lead times for results. These tests include clinicopathological factors and have been demonstrated to improve risk prediction.13-16 Both of these second-generation assays are CE-marked in Europe, but only Prosigna is U.S. Food and Drug Administration (FDA)-cleared for decentralised use in the USA. More detailed information for both first and second-generation multigene prognostic assays developed for use in early-stage breast cancer is presented in Table 1.
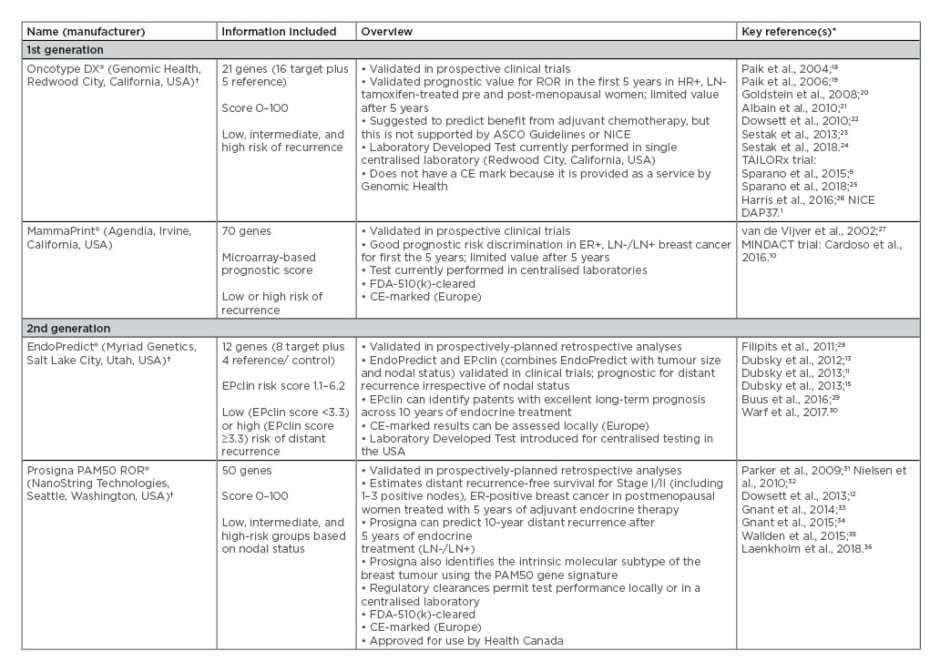
Table 1: First and second-generation multigene prognostic commercial assays developed for use in early-stage breast cancer and were included in the National Institute for Health and Care Excellence evaluation of Prosigna.17
*Relevant company/multigene assay websites used to source some information
†Oncotype DX, Prosigna, and EndoPredict are now funded in Ontario, Canada37
ASCO: American Society of Clinical Oncology; CE: Conformité Européenne; ER: oestrogen receptor; FDA: U.S. Food and Drug Administration; FFPE: formalin-fixed paraffin-embedded; HR: hormone receptor; LN: lymph node; NICE: National Institute for Health and Care Excellence; ROR: risk of recurrence.
The Prosigna 50-Gene Risk of Recurrence Assay
Prosigna is an FDA-510(k)-cleared, Health Canada-approved, and CE-marked GEP assay developed to guide adjuvant chemotherapy decisions in patients with early-stage ER+/HER2- breast cancer, and it can predict recurrence-free survival at 10 years after initiation of treatment.12,31-35 The assay assumes 5 years of endocrine therapy and measures the mRNA expression of 50 genes in formalin-fixed paraffin-embedded tumour tissues, with the signature also including tumour size and nodal status.17 The 50 genes are compared with prototypical gene expression profiles of the intrinsic breast cancer subtypes to identify the intrinsic molecular subtype of the tumour. Four of the 50 genes were shown not to add prognostic accuracy to the assay.35 Therefore, a simplified 46-gene subset is used in combination with a proliferation score derived from the expression levels of 18 of the 46 genes and gross tumour size to produce a Prosigna score. Modelled risk curves are provided for each nodal status separately (node-negative and 1–3 nodes positive).
Hence, the combined signature allows intrinsic subtype classification and prediction of the risk of distant recurrence at 10 years from diagnosis, and can be used as a continuous score (0–100) to provide individualised estimates of distant recurrence expressed as a percentage, or further categorised into low (lymph node negative [LN-] ≤40; lymph node positive [LN+] ≤15), intermediate (LN- 41–60; LN+ 16–40), and high (LN- 61–100; LN+ 41–100) risk groups based on nodal status.38 The Prosigna assay can be performed in local laboratories.
The prognostic significance of Prosigna was clinically validated in the ATAC and ABCSG-8 clinical studies,12,33 and a Danish cohort study (DBCG)36 of endocrine-treated women with early-stage breast cancer. Additionally, a combined analysis of the ABCSG-8 and TransATAC studies confirmed the value of Prosigna for predicting distant recurrence after long-term follow-up.14
The prognostic value of the Prosigna PAM50 ROR score, Oncotype Dx recurrence score, Breast Cancer Index, EndoPredict, CTS, and IHC4 were evaluated in a pre-planned, retrospective biomarker analysis of the ATAC trial in 774 post-menopausal women with early ER+/HER2- breast cancer who had received endocrine therapy for 5 years.24 In patients with node negative disease, Prosigna provided the most prognostic information during Years 0–10 (hazard ratio [HR]: 2.56; 95% confidence interval [CI]: 1.96–3.35), followed by Breast Cancer Index (HR: 2.46; 95% CI: 1.88–3.23) and EndoPredict (HR: 2.14; 95% CI: 1.71–2.68), and Prosigna also provided the most prognostic information during Years 5–10 (HR: 2.77; 95% CI: 1.93–3.96). In patients with node positive disease, EndoPredict provided the most prognostic value for late distant recurrence (HR: 1.87; 95% CI: 1.27–2.76), followed by Prosigna (HR: 1.65; 95% CI: 1.08–2.51).24
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE EVALUATION OF GENE EXPRESSION PROFILING TESTS IN ER+ /HER2- EARLY-STAGE BREAST CANCER
NICE provides national guidance and advice to improve health and social care, and technology appraisals guidance to ensure that the UK National Health Service (NHS) can adopt clinically and cost-effective technologies rapidly and consistently.39 NICE is internationally recognised as a role model for the implementation of cost-effectiveness analyses. A recent NICE evaluation assessed whether tumour profiling tests to guide adjuvant-chemotherapy decisions in patients with early-stage breast cancer are clinically and cost-effective. Five tumour profiling tests were included in the evaluation: EndoPredict, Oncotype DX, MammaPrint, IHC4, and Prosigna.17,40 IHC4 has not been analytically validated or developed as a commercial product.
Two methods were employed for the evaluation: a systematic review of available literature and a review of existing economic analyses. In addition, a de novo health-economic model was used to assess the cost-effectiveness of the five tests. The searches were performed in February–March 2017, and search strings combined synonyms for ‘breast cancer’ with individually named tumour profiling tests.17
The systematic literature review included a search of 14 electronic databases and trial registries, plus ad-hoc supplementary searches. Included studies assessed the clinical effectiveness of the five tests, with a focus on patients with ER+/HER2- early-stage breast cancer with 0–3 positive lymph nodes. Of 533 full-text articles assessed for eligibility, 153 articles were included in the assessment report; 380 studies were excluded from the analysis for a number of reasons: the population, intervention (e.g., neoadjuvant studies excluded), comparator, outcome, or study type were not relevant; could not obtain full text; or there were no novel data. Outcomes included prognostic performance, prediction of chemotherapy benefit, clinical utility, and decision impact.17
For the economic review, 26 studies were identified that assessed the cost-effectiveness of tumour profiling tests in the UK, USA, Canada, Mexico, Japan, Austria, Germany, France, and the Netherlands. None of the studies identified in the literature review included all of the tests; therefore, a de novo economic model was developed to assess the cost-effectiveness of the five tests compared with current practice, where tumour profiling tests are not used. This model used a bespoke analysis of the TransATAC trial as the main source of evidence to inform the parameters. As MammaPrint was not included in TransATAC, the MINDACT trial was used as the basis for evaluating the cost-effectiveness of this test.17
The current European standards of care for prediction are PREDICT,41,42 Nottingham Prognostic Index (NPI),43,44 and Adjuvant! Online,45,46 which are web-based clinicopathological tools that use patient and disease characteristics to define a level of clinical risk and aid physicians in prescribing decisions for adjuvant chemotherapy. The use of these tools varies between centres, but currently PREDICT is thought to be the most widely used tool in the NHS and Adjuvant! Online is not currently available. The NPI score takes into account the size of the tumour, the number of lymph nodes involved and the tumour grade to classify patients into four different risk-group categories to predict 5-year survival.47 For LN- disease, the NPI score is based only on tumour grade and size: NPI ≤3.4 is classed as low risk and NPI >3.4 is classed as intermediate risk. PREDICT scores were not available for the NICE evaluation, so standard current practice (other tools and algorithms) was used as the comparator for Oncotype DX, Prosigna, IHC4+clinopathological factors (IHC4+C), and EndoPredict. The comparator for MammaPrint was a modified version of Adjuvant! Online.17
In the NICE evaluation, the prognostic ability of the tests was preferred over predictive value as certain breast cancer subtypes are prone to distant recurrence despite chemotherapy treatment. Limited data were available that evaluated the ability of Oncotype DX and MammaPrint to predict benefit from chemotherapy, and some analyses were also open to criticisms relating to adjustment for all relevant variables.17 Evidence to support Oncotype DX’s ability to predict a benefit from chemotherapy was considered weak because adjusted interaction tests were not always statistically significant, particularly in the LN+ group.17
Clinical Effectiveness of Prosigna
Eight data sets (N=9,118) were used to assess Prosigna’s prognostic performance, including six reanalyses of randomised clinical trial data (TransATAC, ABCSG-8, CALGB 9741, NCIC MA.21, GEICAM 990683, and NCIC MA.12) and two retrospective analyses of prospective cohort studies (DBCG and the British Columbia cohort).17
In unadjusted analyses, patients with either LN- or LN+ disease evaluated using the Prosigna test had statistically significant prognostic accuracy for 10-year distant recurrence-free survival and distant recurrence-free interval.17 In adjusted analyses, Prosigna improved prognostic accuracy over clinical and pathological variables or prediction tools alone.17 In patients with LN- disease, Prosigna combined with CTS or NPI significantly improved prognostic accuracy versus CTS or NPI alone (p<0.0001 for both). Similar results were seen in LN+ patients, although significance was borderline (Prosigna+CTS versus CTS alone, p=0.04; Prosigna+NPI versus NPI alone, p=0.09).17 In the NICE evaluation, the evidence suggested that all of the tumour profiling tests have the ability to predict the risk of distant recurrence in the population included in the assessment, although the evidence was weaker for LN-positive disease than LN-negative disease.17
Cost-Effectiveness of Prosigna versus Other Tests
A de novo hybrid decision tree–Markov model was designed to assess the cost-effectiveness of the tests compared with current practice without the use of tumour profiling tests, using a lifetime time horizon (42 years) from the perspective of the NHS and personal social services. Patients were assumed to enter the model aged 58 years and the evaluation continued until the cohort reaches age 100 years. All costs and health outcomes were discounted at a rate of 3.5% per year. Unit costs were valued at 2015–2016 prices. The main source of evidence used to inform the analyses of Oncotype DX, Prosigna, IHC4+C, and EndoPredict was a bespoke analysis of TransATAC, which was limited to UK data on patients with HR+/HER2- disease with 0–3 positive lymph nodes. MINDACT was used as the basis for evaluating the cost-effectiveness of MammaPrint.17
The decision tree component of the model classified patients in the current practice group (no test) and the tumour profiling test group as high, intermediate, and low risk. The treatment effect for adjuvant chemotherapy was modelled using a relative risk reduction for distant recurrence within each risk classification group. The benefit of the test was therefore captured in the model by changing the probability that patients with each test risk classification had adjuvant chemotherapy. Six patient groups and four health states were considered. Costs of endocrine therapy, follow-up visits, mammograms, treating local recurrence, and distant metastases were factored into the model. Costs associated with terminal care were excluded.
For the Prosigna test compared with current practice, the cost-effectiveness analysis reported incremental cost-effectiveness ratios (ICER) of £91,028 (LN-, NPI ≤3.4), £26,058 (LN-, NPI >3.4), and £28,731 (LN+) per quality-adjusted life-year (QALY) gained. In LN- disease, the Prosigna ICER was lower compared with EndoPredict and Oncotype DX, but higher versus IHC4+C (Table 2).
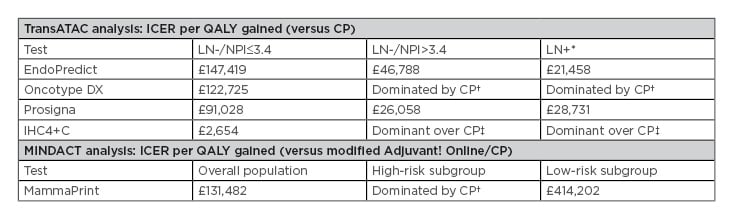
Table 2: National Institute for Health and Care Excellence cost-effectiveness analysis of tumour profiling tests used at list price to guide adjuvant chemotherapy decisions in early-stage breast cancer.17
*Note that in patients with ER+/HER2-/LN+ early-stage breast cancer, none of the tests were recommended by NICE as options for guiding adjuvant chemotherapy decisions.
†More expensive, less effective
‡Less expensive, more effective
CP: current practice; ICER: incremental cost-effectiveness ratios; IHC4+C: 4-marker immunohistochemical score plus clinicopathological factors; LN: lymph node; NICE: National Institute for Health and Care Excellence; NPI: Nottingham Prognostic Index; QALY: quality-adjusted life-years.
In LN+ disease, the Prosigna ICER was higher compared with EndoPredict and IHC4+C.17 It should be noted, however, that not all tests have the same prognostic value.
In the LN-/NPI >3.4 group of patients, at a maximum acceptable ICER of £20,000 and £30,000 per QALY gained, Prosigna (at list price) had a probability of 0.24 and 0.60, respectively, of being cost-effective. In the LN+ group of patients, at a maximum acceptable ICER of £20,000 and £30,000 per QALY gained, Prosigna had a probability of 0.24 and 0.55, respectively, of being cost-effective.17
The committee subsequently considered confidential access proposals for Prosigna and EndoPredict. Compared with current practice, ICER for EndoPredict and Prosigna in the LN-/NPI ≤3.4 group remained higher than those considered to be cost-effective. However, in the LN-/NPI >3.4 group, Prosigna compared with current practice had an ICER of <£20,000 per QALY gained, and was therefore considered cost-effective, whereas EndoPredict had ICER from £20,000–30,000 per QALY gained depending on local versus centralised laboratory testing.17
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE RECOMMENDATION
Overall, Prosigna, EndoPredict, and Oncotype DX Breast Recurrence Score were recommended by NICE as options for guiding adjuvant chemotherapy decisions for patients with ER+/HER2-/LN- early-stage breast cancer.17 None of the tests were recommended for use in LN+ disease. NICE recommended the Prosigna test based on the above analyses; Prosigna was statistically significantly prognostic for unadjusted analyses of 10-year distant recurrence-free survival and distant recurrence-free interval in LN- and LN+ patients and, in adjusted analyses, the test added prognostic information over clinicopathological variables, which was statistically significant in LN- patients. However, the committee concluded that none of the tests had strong enough evidence to demonstrate an effect on subsequent patient outcomes. In the LN-/NPI >3.4 group, Prosigna compared with current practice had an ICER of <£20,000 per QALY gained when considering a confidential access proposal and was considered cost-effective, but evidence on clinical outcomes will be important to confirm this.17 The NICE committee noted that when evaluating the tests, there were several ongoing studies which will provide evidence of long-term outcomes: MINDACT (MammaPrint), TAILORx (Oncotype Dx), and OPTIMA (Prosigna). Data from these studies will be important for future updates to the guidance. In fact, data from the TAILORx study were published in 2018,25 after the original NICE analysis, which delayed release of the final DG34 recommendations.40 The final clinical and cost-effectiveness outcomes in the NICE study remained unchanged in light of the new data, and it should be noted that patients evaluated in the NICE analysis were of substantially higher clinical risk compared with those in the TAILORx study. Similarly, based on data from the MINDACT study,10 the clinical utility of MammaPrint was also considered by NICE. However, the authors of this study confirmed that long-term data are essential, and NICE concluded that there is not enough evidence to demonstrate an effect on patient outcomes. It should also be noted that not all studies will provide UK-specific data and comparisons with the PREDICT tool, which are important to fully assess the cost-effectiveness of the tests compared with current practice.
EVALUATION AND RECOMMENDATION OF REGULATORY BODIES
It is important to note that clinical and cost-effectiveness analyses (such as those provided by NICE), evaluations by local regulatory bodies, and inclusion in clinical practice guidelines are required for widespread use of GEP tests in many countries. American Society of Clinical Oncology (ASCO),26 National Comprehensive Cancer Network (NCCN),3 European Society for Medical Oncology (ESMO),2 St Gallen,48 Spanish Society of Medical Oncology (SEOM),49 and German Gynecological Oncology Group (AGO)50 guidelines all recommend the use of prognostic multigene assays, including Prosigna, in early-stage breast cancer; further details are provided in Table 3.
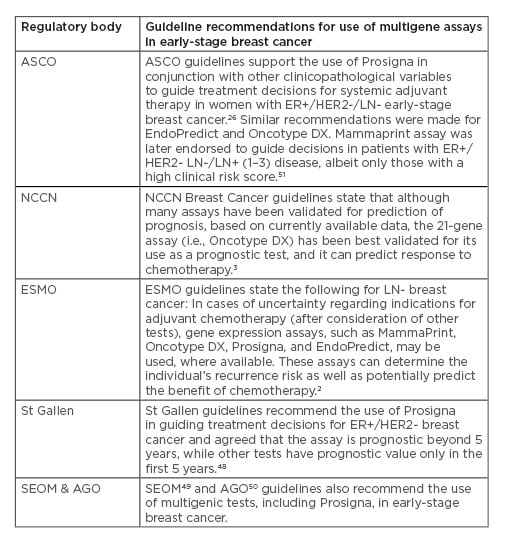
Table 3: Guidelines supporting the use of Prosigna and other multigene assays to guide adjuvant chemotherapy decisions in early-stage breast cancer.
AGO: German Gynecological Oncology Group; ASCO: American Society of Clinical Oncology; ER: endocrine receptor; ESMO: European Society for Medical Oncology; HER2: human epidermal growth factor 2; LN: lymph node; NCCN: National Comprehensive Cancer Network; SEOM: Spanish Society of Medical Oncology.
These tests are recommended in treatment guidelines based on the additional prognostic information they provide.
ONGOING RESEARCH EFFORTS TO ADDRESS REMAINING UNCERTAINTY
Ongoing Research Studies Evaluating Prosigna: UK OPTIMA Study
The ongoing OPTIMA trial of Prosigna, a randomised controlled trial with a projected recruitment of 4,500 patients in the UK, will provide evidence of long-term patient outcomes following Prosigna scoring.52 The study aims to validate use of the assay to help guide clinical decisions for adjuvant chemotherapy for patients with hormone-sensitive, HER2- and LN+ (up to nine nodes) early-stage breast cancer52 and will provide valuable information for future updates to the NICE evaluation of GEP tests in early breast cancer, which currently does not recommend any GEP tests for LN+ disease.17
In this clinical utility study, two groups of patients will be randomised to be assessed with the Prosigna test or not. In the group assessed using Prosigna, therapy decisions will be guided by the results of the test. In the untested group, patients will receive adjuvant chemotherapy followed by hormone therapy. OPTIMA may help to reduce uncertainties surrounding the potential utility of the test in patients with LN+ breast cancer, particularly in patients who have comorbidities and may have further negative impacts from the side effects associated with adjuvant chemotherapy.
To establish priorities for further cost-effectiveness of multiparameter assays in the NHS, an economic modified Markov model was used in the preliminary phase to estimate mean differences in clinical effects (including life years and QALY) and costs using Adjuvant! Online or constant and variable benefit models in accordance with the NICE reference case. The base-case analysis included Oncotype DX, MammaPrint, and Prosigna but excluded IHC4, MammaTyper, and IHC4 AQUA because test cost and evidence for analytical and clinical validity were not available for all tests.52
When using a willingness-to-pay threshold of £20,000 per QALY, the probability of the tests being more cost-effective than chemotherapy for all patients ranged from 77% (MammaPrint) to 79% (Oncotype DX and Prosigna). The probability of tests being cost saving versus chemotherapy for all patients was 39% for MammaPrint, 53% for Oncotype DX, and 68% for Prosigna.52
From an NHS perspective, the value of information for further research into Prosigna was considered higher than for the two other assays that were evaluated in this study. There is considerable uncertainty regarding the cost-effectiveness of the tests, which is attributable not only to their performance but also their long-term outcomes. Further sensitivity analyses are required to better understand which model parameters drive costs and value of information.52
Other Key Studies Evaluating Gene Expression Profiling Tests in Early-Stage Breast Cancer
Prospective studies evaluating other GEP tests in ER+/HER2- early-stage breast cancer are ongoing. An evaluation of MammaPrint in the randomised Phase III trial MINDACT (N=6,693) suggested that in women at high clinical risk and low genomic risk for recurrence, the 5-year rate of survival without distant metastasis in patients receiving no chemotherapy, determined on the basis of the test, was only 1.5% lower than in patients receiving chemotherapy. It was therefore suggested that chemotherapy may not be required in approximately 46% of women with high-risk breast cancer.10
In the Phase III TAILORx study, both endocrine therapy and chemoendocrine therapy had similar efficacy in ER+/HER2-/N0 patients with a midrange recurrence score as determined by the Oncotype DX assay.25 Also, patients with LN+ disease and low-to-intermediate Oncotype DX recurrence scores in the ongoing RxPONDER study53 will be randomised to hormone therapy with or without chemotherapy. The study’s results are eagerly anticipated.
CONCLUSION AND FUTURE DIRECTIONS
Patients with certain subtypes of breast cancer may not benefit from adjuvant chemotherapy following surgery to remove the primary tumour. Molecular-level analysis of tumour tissue has enabled the identification of four main subtypes of breast cancer, which each have distinct gene expression patterns. This information enables patients to be stratified according to those who are likely to benefit from chemotherapy and those who are not, which may ultimately help to avoid unnecessary treatment and the associated side effects in some patients.
The Prosigna test has been recommended by health authorities and is included in all major treatment guidelines as a diagnostic assay to help guide adjuvant chemotherapy treatment decisions in patients with ER+/HER2-/LN- early-stage breast cancer. The NICE evaluation included a review of the clinical and cost-effectiveness of five tumour-profiling tests, including Prosigna, to make their recommendation. The ongoing OPTIMA trial will provide further information on the effectiveness of Prosigna for guiding treatment decisions in patients with LN+ disease.
There is also uncertainty regarding the extension of hormone therapy beyond 5 years, although data is stronger for extended treatment with tamoxifen compared with an aromatase inhibitor,54 and prognostic tools are now available which can estimate the risk of late distant recurrence in women with ER+ breast cancer.55
There is an unmet need for prognostic signatures that are clinically validated in ER- breast cancers. Further research is warranted to facilitate a personalised medicine approach to treatment and eliminate unnecessary drug exposure in women who would not benefit from chemotherapy or extended endocrine treatment.








