Abstract
Spontaneous carotid artery dissection (SCAD) is a common cause of ischaemic stroke in young patients. Though SCAD is usually defined as carotid artery dissection without trauma, a minor traumatic event is often revealed by a detailed medical interview. An association with intrinsic vessel structure has been reported in connective tissue disease, pregnancy and the postpartum period, and in infectious and inflammatory disease. Mechanisms of SCAD vary and are still unclear, but a combination of fragile vessel walls and minor trauma can result in dissection. Headache, Horner’s syndrome, transient ischaemic attack or ischaemic stroke are the most frequently seen clinical symptoms. Non-invasive diagnostic modalities including magnetic resonance imaging, computed tomography, and duplex ultrasonography have become an alternative to digital subtraction angiography, however this still remains the gold standard, with better detection of thrombus and collateral circulation. Antithrombotic therapy is the standard medical treatment for SCAD, achieving a good clinical course in most patients. Patients, including those on antithrombotic therapy, who are presenting with recurrent ischaemic symptoms, haemodynamic compromise, or expanding pseudoaneurysm should be considered for intervention therapy. As the main purpose of endovascular treatment is to avoid recurrence of ischaemic attack, performing the procedure under an embolic embolic protection device is preferable. To obtain sufficient blood flow and sealing of the thrombus, a stent is usually delivered, and coil embolisation can be performed for expanding pseudoaneurysm, if required. The most common regimen of post-procedural antiplatelet therapy is a double dose for 3 months followed by a single dose indefinitely, though a clear guideline does not exist. The clinical success rate and long-term results of endovascular treatment are acceptable. Endovascular treatment for carotid artery dissection is feasible, safe, and effective in a subgroup of patients, including those resistant to medical therapy.
INTRODUCTION
Although uncommon in the general population, spontaneous carotid artery dissection (SCAD) is a common cause of ischaemic stroke in patients <50 years old, accounting for 14–20% of cases.1SCAD was first reported in 1959.2 Carotid artery dissection can be divided into traumatic, spontaneous, and iatrogenic causes. When the patient does not have any obvious trauma the dissection is typically recognised as spontaneous, however certain minor events including rotation of the neck, coughing, and vomiting, precede dissection in 12–34% of cases.3 Connective tissue diseases inducing intrinsic vessel structure abnormalities, such as Marfan’s syndrome, Ehlers–Danlos syndrome,4cystic medial necrosis, polycystic kidney disease, osteogenesis imperfecta,5 and fibromuscular ysplasia,6,7 are also associated with spontaneous arterial dissection. In some case reports, SCAD was seen in pregnancy and the postpartum period.8-13 Infectious and inflammatory disease may also be causative.14,15 Although our understanding of the various mechanisms remains incomplete, it is clear that a combination of structural fragility in a carotid artery and minor trauma can induce SCAD.
CAROTID ARTERY DISSECTION
Pathophysiology
When a tear develops at the intima or media of an artery, dissection occurs resulting in the flow of blood into the dissected lumen. Ischaemic events are often seen in patients with SCAD. They are caused by thrombi formed as a result of intimal damage and turbulent flow, or by enlargement of the dissected lumen, which leads to stenosis or occlusion of the true lumen. In some cases, a pseudoaneurysm can be seen depending on the depth of the tear. SCAD commonly occurs about 3 cm distal to the origin of the internal carotid artery (ICA)16,17 and intracranial dissection of the ICA is seen in 20% of cases.
Clinical Features
SCAD can cause a variety of symptoms, however headache, Horner’s syndrome, and ischaemic stroke are the most common. Headache occurs in ~70% of SCAD cases18 and neck pain is also seen in 26% cases.19 Horner’s syndrome is induced by damage to the sympathetic pathway distal to the superior cervical ganglion and is seen in 28–58% of cases.20-22 Transient ischaemic attack or ischaemic stroke are seen in 67% of patients with SCAD as a consequence of artery-to-artery thromboembolism from the site of dissection.23
Diagnosis
Digital subtraction angiography (DSA) remains the gold standard modality for the radiographic diagnosis of SCAD, however alternative non-invasive modalities including magnetic resonance imaging (MRI), computed tomography, and duplex ultrasonography are available. DSA allows better evaluation of the thrombus and collateral circulation in haemodynamically-compromised situations. Obvious disadvantages are the time and expense of the procedure and the risks and discomfort to the patient. MRI and magnetic resonance angiography (MRA) can be viable alternatives to DSA and give information regarding ischaemic damage to the brain. MRI, however, has a limited sensitivity to identify an intraluminal thrombus and intramural haematoma, often overestimating the degree of stenosis. The sensitivity and specificity of computed tomography angiography (CTA) in detecting the dissections are similar to DSA and superior to MRA. The ease and speed of use are the primary advantages of CTA, however the problems with this modality are the exposure to contrast and radiation, and the lack of information about brain damage. Duplex ultrasonography is a less invasive imaging modality, but the quality of images is highly dependent on operator experience. Although there is a limited ability to evaluate lesions located more caudally and a tendency to mistake subtotal stenosis for occlusion, duplex ultrasonography remains a relatively suitable modality for follow-up investigations.
Treatment
Medical treatment with antithrombotic therapy is the standard because most ischaemic events are induced by thrombus. In many cases, the patients with SCAD usually have an acceptable clinical course with medical therapy alone. It remains unclear whether anticoagulant or antiplatelet agents provide better antithrombotic effects in this context.6,24,25 The European Cervical Artery Dissections and Ischemic Stroke Patient trial recommends treatment with antiplatelet drugs, except in situations favouring anticoagulation, when recurrent events while on antiplatelet drugs occur, or in the presence of a thrombus.26 Although first reported in 1977, and despite numerous case reports and series, endovascular treatment for SCAD remains controversial due to benign outcomes with medical therapy.27-30 There are no clear guidelines regarding endovascular treatment, however in the following situations endovascular treatment must be considered as an option: 1) patients with recurrent symptoms despite medical therapy; 2) patients with haemodynamic hypoperfusion; 3) patients with an expanding or symptomatic pseudoaneurysm; 4) contraindication to anticoagulation because of intracranial or systemic haemorrhage.31The indication of surgical treatment is limited to the situations described in Table 1, due to the high rates of stroke and cranial nerve injuries. Recurrence of SCAD is rare, with yearly rates reported at ~1%.32-34 Recurrent dissections tend to occur in a different vessel to the previously dissected vessel.35,36
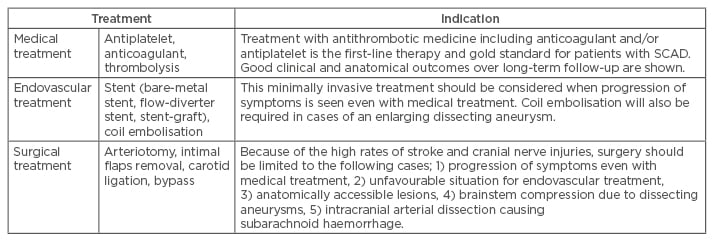
Table 1: Treatment options for spontaneous carotid artery dissection and its indications.
SCAD: spontaneous carotid artery dissection.
ENDOVASCULAR TREATMENT
Endovascular treatment for carotid artery dissection is uniquely challenging. The aims are to restore an adequate antegrade flow to prevent flow-limiting dissection, and to provide an adequate sealing of thrombus avoiding further dislodgment. The technical success rate of endovascular treatment has been reported as 99.1–100% with an overall rate of adverse events of 4–11% and no mortality. Intimal hyperplasia, in-stent restenosis, or occlusion of a treated vessel is seen in 3.3–8% of follow-up cases.37,38
Embolic Protection
To be effective, endovascular treatment of carotid artery dissection needs an adequate technique to protect the brain during the procedure, preventing thrombi dislodgement and embolisation, and an adequate technique to exclude the risk of pseudolumen or pseudoaneurysm. The former could be achieved by neuroprotection devices such as distal filters, or proximal protection with flow-blockage or inversion. Proximal protection allows complete protection during all phases of carotid artery stenting, as well as during lesion crossing with the wire. It has shown higher efficacy than distal filters in reducing silent cerebral embolisation and it should be the first choice in treating symptomatic patients.39
How to Ensure Correct Location of a Guide Wire
There are some difficult aspects to treating a dissected carotid artery. In particular, it is often challenging to advance the guide wire into the true lumen whilst avoiding the dissected lumen. To confirm that the guide wire is inside the true lumen, the the following techniques are recommended: 1) compare the angiography carefully; 2) use intravascular ultrasound; 3) tip injection by microcatheter.40
Stenting and Coil Embolisation
With regard to the type of stent, several techniques have been used to treat the dissected carotid artery and dissecting aneurysm:
- Bare-metal stent: A small diameter, balloon-expandable stent such as a coronary stent is often used for the distal and petrous portions because of the small diameter of the vessel. A self-expandable stent is usually used for the proximal and ostial portions of the ICA due to the continuous radial force toward the vessel wall. Two or more overlapping carotid stents can be used to increase the mesh density, preventing clot migration and leading to secondary occlusion of the pseudolumen and peusdoaneurysm29
- Stent-assisted coil embolisation: Coils are deployed under the stent struts, using the stent as a scaffold that prevents coil migration and dispersion; incomplete occlusion with recanalisation has been reported in up to 20% of cases41
- Flow-diverter stent: Aneurysm obliteration can be achieved using a flexible, self- expanding nitinol stent with platinum microfilaments specifically designed to produce a haemodynamic flow diversion and to reconstruct laminar flow in the parent artery42
- Stent-grafts: Commonly used to repair vascular lacerations, a covered stent deployed across the neck of the pseudoaneurysm could easilyfix this problem.43,44 Navigation of the stent-grafts through the vascular loops can be challenging due to the rigidity of the system, particularly through tortuous, small vessels, or the distal internal carotid artery near the carotid siphon. This may lead to vasospasm, propagation of the existing dissection or or new dissection at the ends of the stent, and even vessel rupture. Newly available self-expandable stent-grafts are more flexible and have a lower profile to overcome delivery problems. Stent-grafts may have a higher rate of thrombosis and restenosis compared to bare-metal stents
Antiplatelet Therapy after the Procedure
The optimal regimen of post-procedural antiplatelet therapy is yet to be defined. Double antiplatelet therapy is typically used but the duration varies between centres from 1–6 months. Most commonly double antiplatelet therapy is completed for 3 months and single antiplatelet therapy was continued indefinitely.
REPRESENTATIVE CASE
A 63-year-old male was referred to the emergency department complaining of right hemiparesis and aphasia (National Institutes of Health Stroke Scale [NIHSS] score of 7). Diffusion weighted MRI (DW-MRI) showed no high intensity spots, but MRA indicated insufficient flow of the left ICA (Figure 1). CTA revealed a left carotid artery dissection (Figure 2). Just after these examinations, his symptoms markedly improved (NIHSS score of 0). The patient started to receive conservative therapy with antithrombotic drugs. The next day, DW-MRI and MRA were performed because of a deterioration in symptoms (NIHSS score of 8), and revealed high intensity spots inside the territory of the middle cerebral artery and decreased blood flow of the left ICA (Figure 1). The patient thus underwent endovascular treatment. Baseline DSA revealed dissection of the left ICA (Figure 2) and intracranial angiography showed insufficient flow to the brain (Figure 3). We advanced a 0.014 inch guide wire into the left ICA distal, confirming that the guide wire was inside the true lumen with intravascular ultrasound. Under an embolic protection device (Angioguard™ XP, Cordis Corp., FL, USA), a Driver® stent 4.0 mm/30.0 mm (Medtronic, MN, USA) was delivered from the distal lesion in order to completely cover the dissected lumen. After that, the Precise® stent 6.0 mm/40.0 mm (Cordis) was delivered for the proximal lesion in an overlap manner (Figure 4 and 5). The final angiogram showed complete reconstruction of the left ICA (Figure 2) and the intracranial DSA showed sufficient flow to the brain (Figure 3). The symptoms were improved immediately after the procedure without any complications.
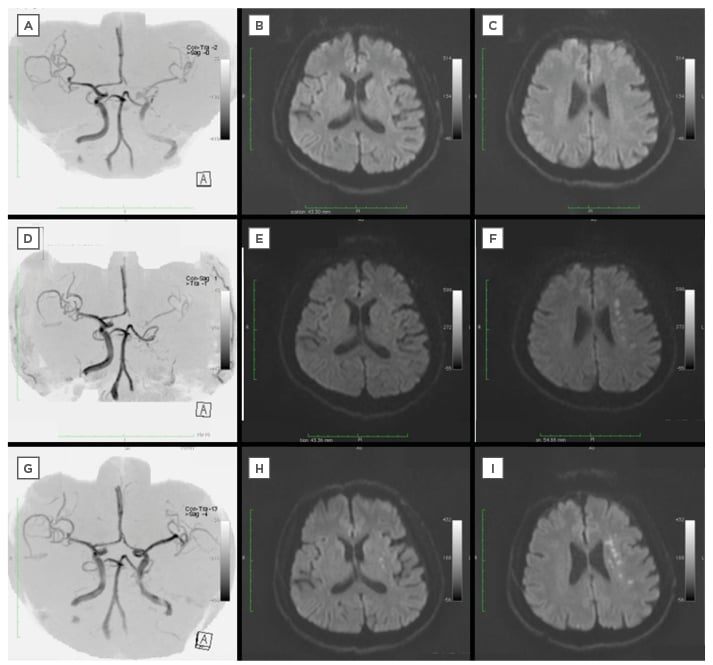
Figure 1: Magnetic resonance imaging of a 63-year-old man admitted with a National Institutes of Health Stroke Scale Score of 7.
A–C: On admission, MRA indicated insufficient flow of left ICA but no HIS in DW-MRI.
D–F: Decreased blood flow of left ICA was seen in MRA and HIS inside the territory of a middle cerebral artery in DW-MRI when the symptom was worsening.
G–I: MRA and DW-MRI performed after endovascular treatment revealed adequate flow of left ICA and no enlargement of HIS.
MRA: magnetic resonance angiography; ICA: internal carotid artery; HIS: high intensity spots; DW-MRI: diffusion weighted magnetic resonance imaging.
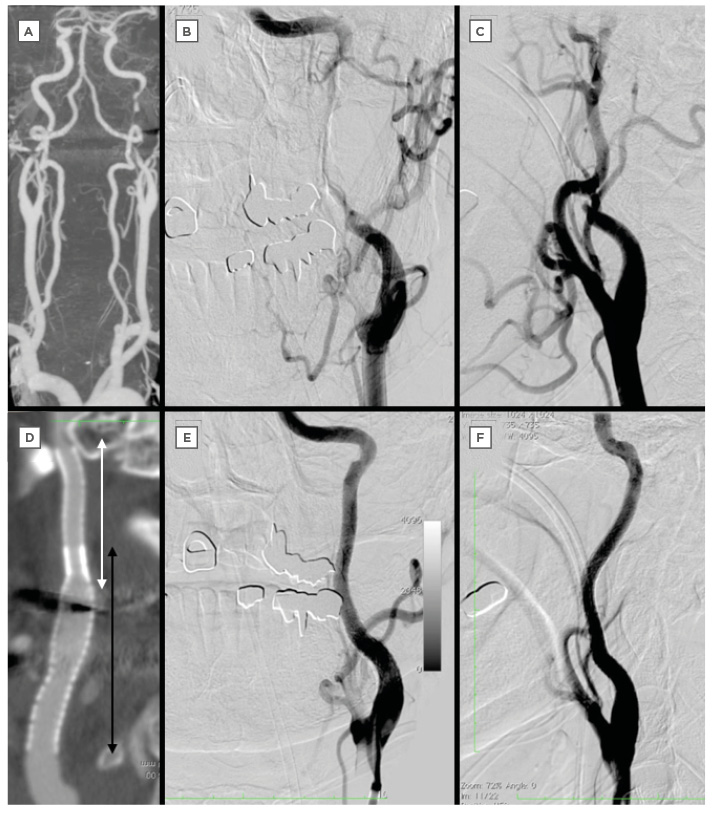
Figure 2: Computed tomography and digital subtraction angiography of a 63-year-old man admitted with a National Institutes of Health Stroke Scale Score of 7.
A–C: CTA and DSA on admission indicate artery dissection of left ICA.
D: CTA after the procedure shows the implanted stents (white double-headed arrow is Driver® stent 4.0 mm/30.0 mm, black double-headed arrow is Precise® stent 6.0 mm/40.0 mm).
E–F: DSA after stenting show complete reconstruction of left ICA.
CTA: computed tomography angiography; DSA: digital subtraction angiography; ICA: internal carotid artery.
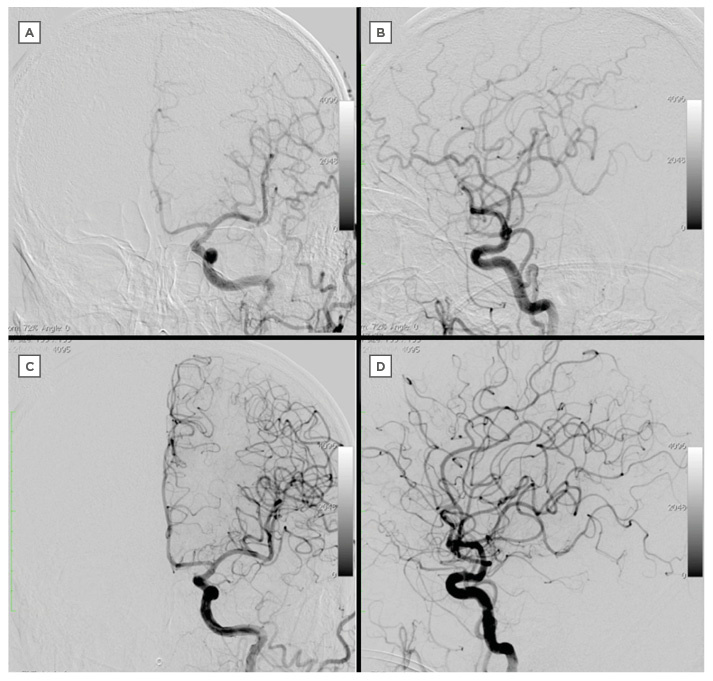
Figure 3: Comparison of intracranial vessel before and after procedure.
A–B: Intracranial DSA on admission.
C–D: Intracranial DSA after the procedure.
DSA: digital subtraction angiography.
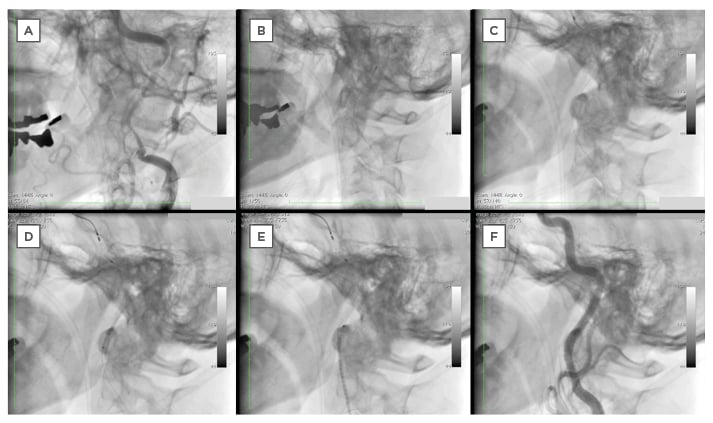
Figure 4: Progression of stent delivery from baseline to complete endovascular treatment.
A: Baseline angiogram.
B: Performing IVUS after advancing the guide wire in order to confirm the guide wire is inside true lumen.
C: Filter placed at the distal end of the left ICA.
D: Delivery of Driver® stent.
E: Delivery of Precise® stent.
F: Final angiogram.
IVUS: intravascular ultrasound; ICA: internal carotid artery.
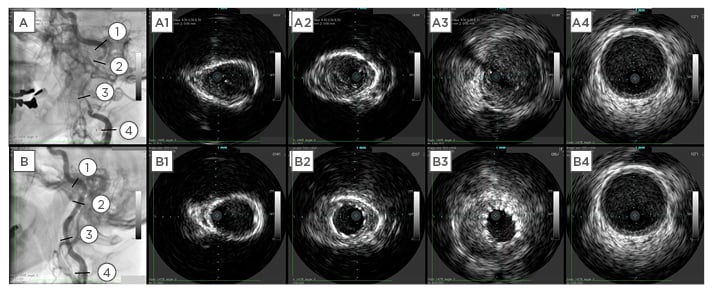
Figure 5: Comparison of intravascular ultrasound images before and after procedure.
A1–A4: Intravascular ultrasound images of the left internal carotid artery before the procedure. B1–B4: Intravascular ultrasound images following stenting.
CONCLUSION
SCAD is the most common cause of ischaemic stroke in younger patients. Preceding minor trauma and intrinsic vessel structure abnormalities are often seen in patients with SCAD. With regard to diagnostic modality, DSA is still the gold standard due to its advantage of detecting intraluminal thrombus. Alternative imaging modalities include MRI, computed tomography and duplex ultrasound, but each of these has its own advantages and drawbacks. Antithrombotic therapy with an anticoagulant or an antiplatelet is essential and most cases are successfully treated with low recurrence rates. For some patients however, such as those resistant to medical therapy, endovascular treatment is a viable option. According to a number of review articles and case reports, endovascular treatment for carotid artery dissection is feasible, safe, and effective in this group of patients.








