INTRODUCTION
There is increasing utilisation of prosthetic orthopedic implantations. These implantations include joint replacements for the knees, hips, and shoulders.1 Post-operative pain or rashes may raise concerns for an underlying allergic process, especially if these complications are associated with prosthetic joint failure.1,2 There is evidence that allergic contact dermatitis (ACD) is a possible complication for implanted devices, but there is conflicting data regarding the efficacy of patch testing for detecting an allergic reaction below the skin surface. Prior studies seem to suggest that pre-operative patch testing can be helpful in guiding device choice in patients with a history of metal allergy, but the role of patch testing in the post-implantation setting is unclear.2,3
This study investigated metal patch testing surrounding orthopaedic device implantation, and whether pre- or postoperative testing results impacted orthopaedic device management.
METHODS
An Institutional Review Board (IRB)-exempt retrospective review was conducted from 2009–2022 of adult patients at a large academic centre who underwent metal patch testing and had procedural codes for knee replacement, hip replacement, and shoulder replacement. The review identified 36 patients who met inclusion criteria (Table 1).
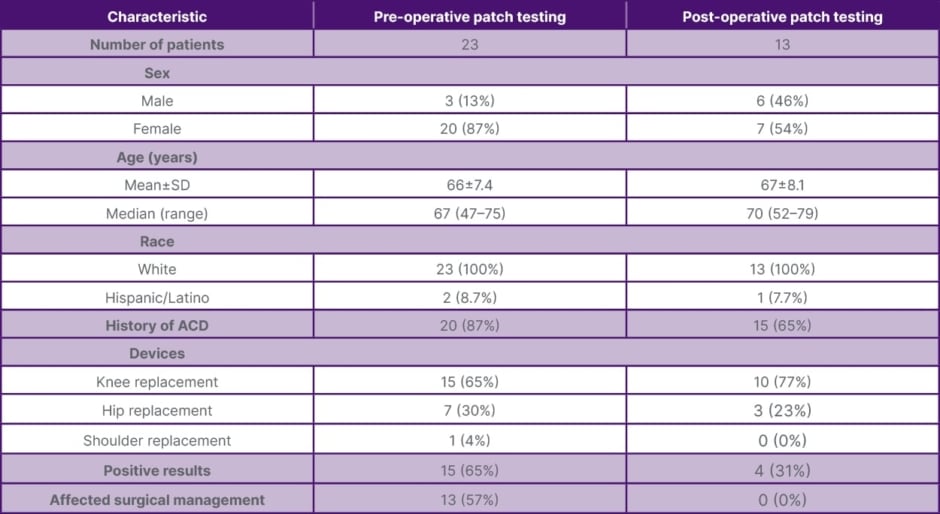
Table 1: Patch testing for joint replacement: demographics, results, and impact (2009–2022).
ACD: allergic contact dermatitis; SD: standard deviation.
RESULTS
Preoperative patch testing was performed for 23 patients who underwent knee replacement (15 patients), hip replacement (seven patients), and shoulder replacement (one patient). Of these patients, 20 had a history of ACD; of the three without a history of ACD, one patient noted a history of oral lichen planus possibly related to his dental implants. In patients who underwent patch testing preoperatively, 15 had clinically relevant positives that impacted selection of joint implant in 13 of those cases; all of these patients had prior history of ACD. Relevant pre-operative patch test positives include nickel sulfate hexahydrate (eight patients), potassium dicyanoaurate (five patients), cobalt chloride hexahydrate (four patients), gold sodium thiosulfate (four patients), manganese chloride (four patients), ferrous chloride (three patients), gold sodium thiosulfate dihydrate (three patients), cobalt sulfate (two patients), potassium dichromate (two patients), chromium chloride (one patient), copper sulfate pentahydrate (one patient), palladium chloride (one patient), ticonium (one patient), and zirconium chloride (one patient).
Postoperative patch testing was performed for 13 patients who underwent knee replacement (10 patients) and hip replacement (three patients). Of these patients, five had a history of ACD. For these postoperative patch testing patients, nine had negative testing while four patients had positive patch testing results of questionable clinical relevance. Implant removal was not recommended in any of these patients.
CONCLUSION
These results suggest that preoperative patch testing can guide orthopedic prosthetic device selection in patients with a history of metal contact allergies, while postoperative patch testing is more limited in utility.






