Interview Summary
Androgen hormones and signalling via the androgen receptor play a key role in regulating the hair cycle and are strongly implicated in the pathogenesis of androgenetic alopecia (AGA). CosmeRNA ARI (ARI, androgen receptor inhibitor) (Bioneer Corp, Seoul, South Korea), which utilises Self-Assembled Micelle Inhibitory RNA (SAMiRNATM) technology represents a distinct approach to treating AGA that is applied topically and selectively targets androgen receptor (AR) mRNA to downregulate overexpression of the androgen receptor. In clinical studies, inhibiting androgen receptor expression using SAMiRNA micelles was shown to be an effective approach to treating AGA that significantly increased hair growth. During interviews conducted by the European Medical Journal (EMJ), two leading experts in the field of hair loss, Sergio Vañó-Galván, Director of the Hair Disorders Unit at Ramón y Cajal University Hospital and Associate Professor of Dermatology at the University of Alcalá in Madrid, Spain, and Young-jin Park from the Yas Clinic, Khalifa City in the UAE, discussed the current and future therapeutic landscape in AGA, and considered the role of SAMiRNA as a promising new treatment approach.THE EVOLVING TREATMENT LANDSCAPE IN ANDROGENETIC ALOPECIA
Current Treatments for Androgenetic Alopecia
AGA represents the most common type of hair loss in men and women, and is driven by both underlying genetic factors and an excessive response to androgens.1,2 As Vañó-Galván explained: “AGA is the most frequent type of alopecia, affecting three out of four men, and one in four women. Clinical presentation consists of thinning of the hair, so patients experience a decrease in hair density. It is not just a hair problem because it can impact quality of life,” he stressed.
Summarising the current treatment landscape in AGA, Vañó-Galván highlighted two main first-line treatments.1,3 “The first one is the antiandrogen treatments that inhibit the effects of androgens, male hormones, which are one of the key causes of AGA. We can use these antiandrogens in an oral way, as topical liquids, or injected. Antiandrogen treatments are the most effective treatment in male AGA,” Vañó-Galván noted. “The second first-line treatment is a drug called minoxidil, which is a vasodilator drug approved for hypertension that can improve hair density and thickening of the hair. It has been used topically for years, but nowadays, the most popular use is as low-dose oral minoxidil. This treatment is probably most effective in female AGA.”
Vañó-Galván continued: “What we usually do is just combine antiandrogens and minoxidil, and we individualise a therapeutic strategy in each patient because success is based on the compliance of treatment. The treatment will only produce a benefit while the patient is using it, and if the patients stop the treatment, we observe a worsening of the AGA.”
He also added that second-line treatments may be useful in certain patients with AGA, “especially those who do not want to take pills or apply topical treatments every day.” These alternative treatment options include mesotherapy, which involves injecting active substances into the scalp, platelet-rich plasma, low-level light therapy, and microneedling.2
The two main oral antiandrogens currently used to treat AGA are finasteride and dutasteride, which act to inhibit the enzyme 5-alpha-reductase that converts testosterone to dihydrotestosterone, thus halting disease progression.4 Both have demonstrated efficacy in the treatment of AGA in clinical trials and real-world studies.2
“The main advantage of conventional treatments is that, in the cohort of patients that have good compliance and tolerate the treatment, these therapies are really effective, and the hair density is really improved. So, the majority can avoid hair transplantation,” said Vañó-Galván. “Another advantage is that we have different modalities of treatment so we can individualise the therapeutic strategy according to the preferences of each patient.”
Looking at the limitations of current treatments, Vañó-Galván outlined three main disadvantages. “The first one is that therapy should be maintained in the long term. This is something that we have to explain to patients: this is not curative; this is a chronic treatment. The second limitation would be the side effects, especially of antiandrogen treatment. With systemic antiandrogens, mainly finasteride or dutasteride, there is a percentage of patients that can develop sexual side effects,” he acknowledged. Vañó-Galván also alluded to a potential ‘nocebo effect’ whereby patients’ worries and concerns about potential sexual adverse events can actually contribute to their occurrence. “And of course, there are other potential side effects,” he continued. “Oral minoxidil can produce hypertrichosis, which is excessive hair growth on the body; lightheadedness because of the decrease in blood pressure; tachycardia; or fluid retention…albeit not very frequently. The third limitation, in my experience, is compliance or adherence, and the frequency of the treatment. For example, finasteride should be taken daily and dutasteride three or four times a week.”
New And Emerging Technologies for Androgenetic Alopecia
Looking to the future, Vañó-Galván remarked that “we are at a good moment for new treatments for AGA because almost every year there are new developments,” as he summarised some of the emerging treatments of interest.
“Botulinum toxin we are starting to use in clinical practice, and it has an advantage, which is that it decreases sweating of the scalp, so patients sense some improvement in the aesthetics of the hair, not only in hair density,” Vañó-Galván noted. “The best dose and frequency are not known, so it is an interesting development, but we have to wait until further research is performed,” he added.
New topical antiandrogens and topical formulations of existing oral antiandrogen agents are also under development, but Vañó-Galván indicated that results of recent clinical trials have been disappointing.2 He also described drugs targeting the prostaglandin pathway, such as the PDG2 antagonist setipiprant, as “interesting” but, based on clinical experience to date, lacking demonstrable efficacy in the treatment of AGA.2
Vañó-Galván went on to introduce what he described as a “new concept of topical antiandrogenic treatment” for AGA, namely the self-assembled, micelle inhibitory RNA. “This treatment is like a next step in antiandrogens and one of the most important new treatments, particularly in men, because it is the androgens which produce the thinning of the hair,” he remarked. “So, inhibiting the action of the androgens is one of the main objectives of treatment of AGA.”
“The self-assembled inhibitory RNA does not actually inhibit the action of the androgens, the hormones, it goes a step forward,” he elaborated. “These are micelles that bind to messenger RNA and inhibit protein synthesis, so they decrease the androgen receptors in dermal papilla cells and sebaceous glands, so it is a new mechanism of action. A separate independent study has shown the effectiveness of this weekly application comparable to the application of finasteride 1 mg daily, which is the classical and effective treatment. So, I think that this is an interesting new development that we are using more and more,” he concluded.
DEEP DIVE INTO THE SAMiRNA TECHNOLOGY
Mechanism of Action
In order to identify a potent SAMiRNA for androgen receptor silencing, over 500 candidates were synthesised and screened, leading to the eventual selection of SAMiRNA-AR68, also known by its brand name CosmeRNA ARI, for clinical development.5 SAMiRNA-AR68 represents a novel type of small interfering RNA (siRNA) micelle that, in contrast to conventional siRNA formulations, does not result in innate immune stimulation.5-8 It consists of a dual-conjugated DNA/RNA heteroduplex that spontaneously assembles to form spherical micelles.5 These SAMiRNA micelles have a size less than 100 nm and a neutral charge, which makes them ideal for delivery into cells.5 Micelles can also benefit from more efficient delivery to hair follicles than small molecules through the pumping effect of massage after application.5 In preclinical testing, SAMiRNA-AR68 was efficiently delivered to human follicle dermal papilla cells and hair follicles, and this potent treatment decreased AR mRNA and selectively suppressed the expression of the receptor protein.5 As Park pointed out, “CosmeRNA is on the cutting edge, positioned as a cosmeceutical.”
Describing how the technology behind SAMiRNA specifically targets and improves AGA, Park explained that siRNAs can play a key role in targeting and silencing androgen receptors. “SAMiRNA is a small-molecule material based on the concept of micelles, allowing it to selectively penetrate hair follicles and travel along the follicular wall to reach dermal papilla cells. This technology offers a significant advantage in safely targeting the androgenic receptors, ultimately rendering them non-functional,” he remarked.
Regarding the efficacy and safety profile of this new technology, Park referred to two double-blind, randomised clinical studies of SAMiRNA-AR68 that have been performed to date. These trials, conducted in both male and female patients with AGA, evaluated two different doses and frequencies of SAMiRNA-AR68.5 In the low-dose study, SAMiRNA-AR68 (0.5 mg/mL) (n=24) or placebo (n=24) was applied topically three times per week for 24 weeks. Significant increases in total hair counts with SAMiRNA-AR68 treatment were confirmed through quantitative analysis using a phototrichogram. However, although photographic assessment revealed increased hair density in the SAMiRNA-AR68 low-dose treatment group as compared with baseline, differences were not statistically significant versus placebo.5
In order to increase both efficacy and user convenience, a high-dose clinical study was also conducted whereby SAMiRNA-AR68 (5 mg/mL) was applied once per week for 24 weeks. This second clinical study enrolled 60 patients with AGA who were randomised (1:1) to either active treatment or placebo. Results demonstrated that, in the high-dose SAMiRNA-AR68 treatment group, total hair counts increased significantly at 24 weeks compared with baseline (4.421%; p<0.001), while there were no significant changes in the placebo group (Figure 1).5 Photographic assessment scores also confirmed a significant improvement in hair density versus baseline at Week 16 and Week 24 in the high-dose SAMiRNA-AR68 group (p<0.05 for both). Notably, the SAMiRNA-AR68 high-dose treatment group showed approximately 240% efficacy compared with the low-dose group at 24 weeks, despite only one application per week.5
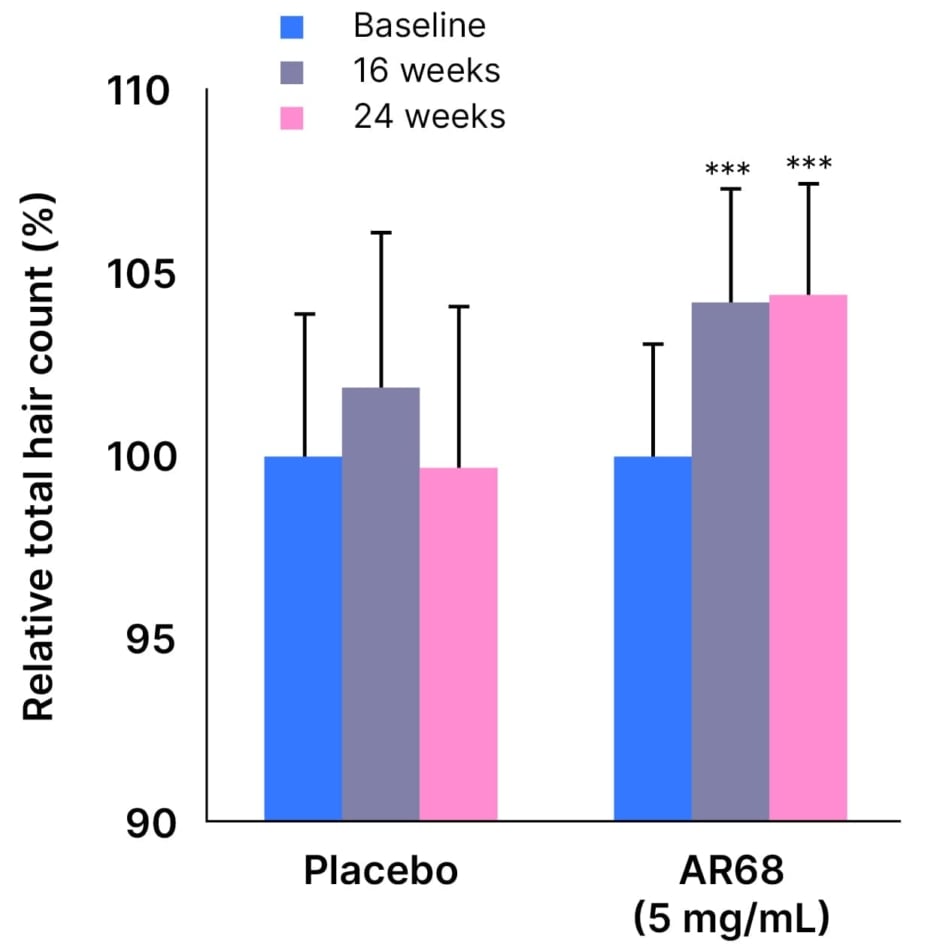
Figure 1: Percentage of total hair count increments at 16 and 24 weeks versus baseline with SAMiRNA-AR68 (5 mg/mL) and placebo.5
***p<0.001 compared with baseline
Adapted from Yun S-I et al. 2022.5
Many of the subjects treated with high-dose SAMiRNA-AR68 in this clinical study also expressed satisfaction in the self-assessment questionnaire. At 24 weeks, the majority of patients (59%) said they were “satisfied with the sample used,” half (50%) described a “feeling of fuller hair in the vertex area,” and nearly three-quarters (73%) reported a decrease in hair loss.5
Based on these results, “I think it is more effective to apply a high-dose less frequently than to apply low doses often,” Park concluded. At the higher dose, SAMiRNA-AR68 demonstrated an average increase in hair growth of 1.3–1.9 hairs per cm2 per month, which is comparable to the efficacy seen with oral finasteride.2,5
SAMiRNA-AR68 also proved well tolerated in both clinical studies and no significant side effects were observed.5 “The micelle technology is biodegraded in the human body,” Park explained. “For the safety assessments, dermatologists observed and assessed subjects for the occurrence of objective irritation, such as oedema, itching, and burning, and recorded details such as start and end date of occurrence, severity, and causal relationship with test product….There were no skin adverse reactions, no skin irritations,” he confirmed. Park also outlined how the SAMiRNA technology has been evaluated in a raft of internal evaluations for safety as a scalp treatment. These tests included assessment of cytotoxicity in dermal papilla cells, HaCaT/innate immune stimulation (evaluating pro-inflammatory cytokines such as IL-1α, IL-1β, and IL-6), and skin penetration. A recent ex vivo experiment performed by Bioneer involved human scalp skin, in which SAMiRNA-AR68 (labelled in red) migrated along the follicular wall to specifically target cells that overexpress androgen receptors within the dermal papilla (Figure 2).
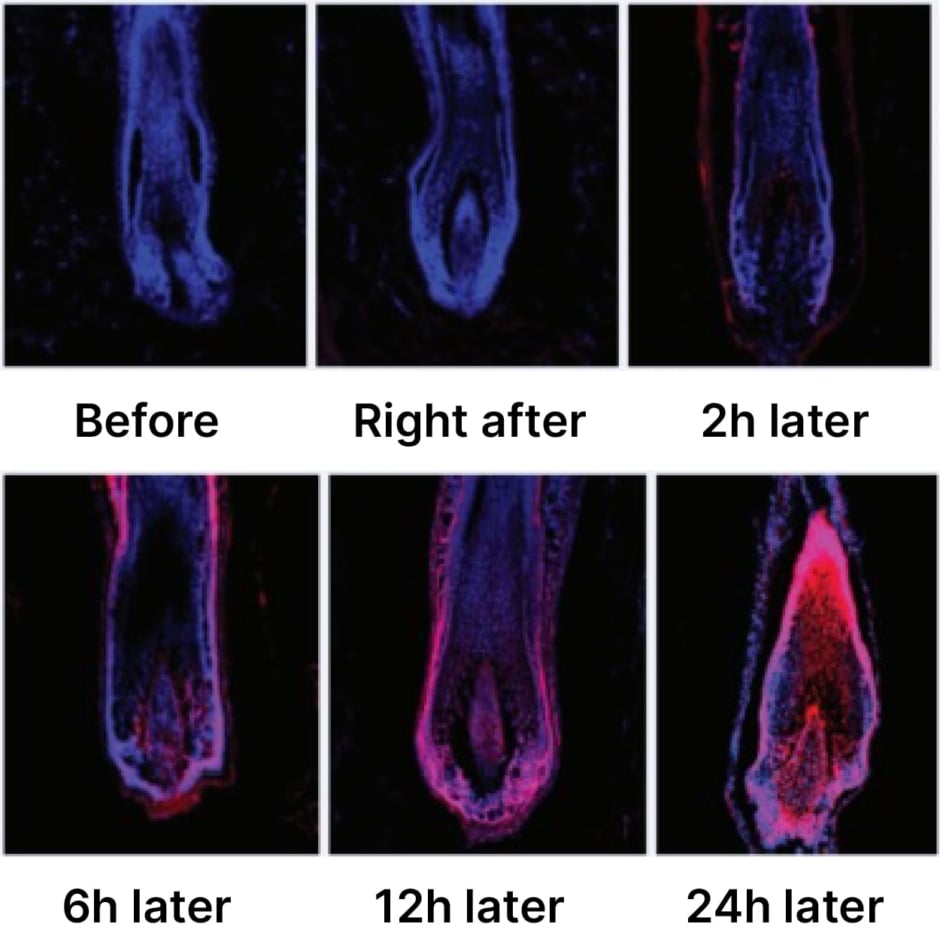
Figure 2: Ex vivo study of SAMiRNA-AR68 in human scalp skin.
“When skin safety was assessed by Dermatest® (Dermatest, Münster, Germany), CosmeRNA also received an excellent five-star rating, so theoretically, practically, and clinically, it can be very safe,” Park concluded. “I have not experienced any complications so far.”
Comparisons with Existing Treatments
Considering how SAMiRNA-AR68, the active ingredient of CosmeRNA, compares with existing treatments in terms of clinical results and patient outcomes, Park noted that “pre-existing drugs like androgenic hormonal blockers such as finasteride and dutasteride can have complications including depression, decreased sexual drive, and feminisation. A significant concern with these antiandrogen hormonal drugs is their potential to cause congenital anomalies if a pregnant woman comes into contact with them,” he added.
“Another alternative is minoxidil,” Park continued, “which works by vasodilation, thereby increasing scalp circulation. While clinical studies show some improvement in female cases, the results are generally less significant compared to antiandrogenic drugs. Additionally, minoxidil may cause contact dermatitis. However, CosmeRNA ARI does not affect hormone levels, and instead it inhibits the overexpression of androgenic receptors. As a result, it can provide more effective results than minoxidil,” he concluded.
Vañó-Galván further explained that, from the patient perspective, a key advantage of existing topical AGA treatments is that they avoid the systemic side effects of oral antiandrogens; however, the compliance of daily application often poses a problem. “I am not an expert yet in SAMiRNA technology but have good experience with it,” he commented. “And probably one of the main advantages of this treatment is that it requires only once a week, or two times per month, application. This is one of the most important advantages when thinking about topical treatments.”
Park highlighted female patients as a specific patient population who may benefit more from SAMiRNA compared to other treatments. “SAMiRNA-AR68 can be particularly beneficial for women dealing with female pattern alopecia associated with hormonal imbalances due to menopause and ageing that can lead to hair loss,” he suggested. “Traditional treatments like dihydrotestosterone blockers are often less effective for female pattern hair loss because this type of hair loss is more closely linked to the overexpression of androgen receptors rather than elevated androgen levels. CosmeRNA ARI addresses this by targeting and normalising the overactive androgenic receptors. As a result, it can offer significant improvements in these cases where hormonal therapies may not provide the desired result.”
REAL-WORLD EXPERIENCE WITH SAMiRNA
Based on his experience in the UAE, Park explained how SAMiRNA has been received by healthcare professionals (HCP). “Initially, CosmeRNA ARI gained traction primarily within clinics, where HCPs began incorporating it into their treatment offerings. However, as we provided more education and assurance about the effectiveness and safety, its popularity has grown significantly. Now, we are seeing increasing interest and adoption among aestheticians, in addition to HCPs, as they recognise the benefits CosmeRNA can offer their clients.”
Park drew attention to one particular patient case study where SAMiRNA technology proved particularly effective (Figure 3).9 “In this patient, the positive effects were maintained for more than 6 months, with application every 2 weeks. Remarkably, even after discontinuing CosmeRNA, the result persisted for over 12 months. This suggests that CosmeRNA’s effectiveness is closely linked to the hair growth cycle, providing long-lasting benefits even after the treatment has ended,” he remarked. siRNA activity is maintained for extended time periods due to the RNA-induced silencing complex in cells, which supports this observation that efficient delivery is more important than frequent applications.5
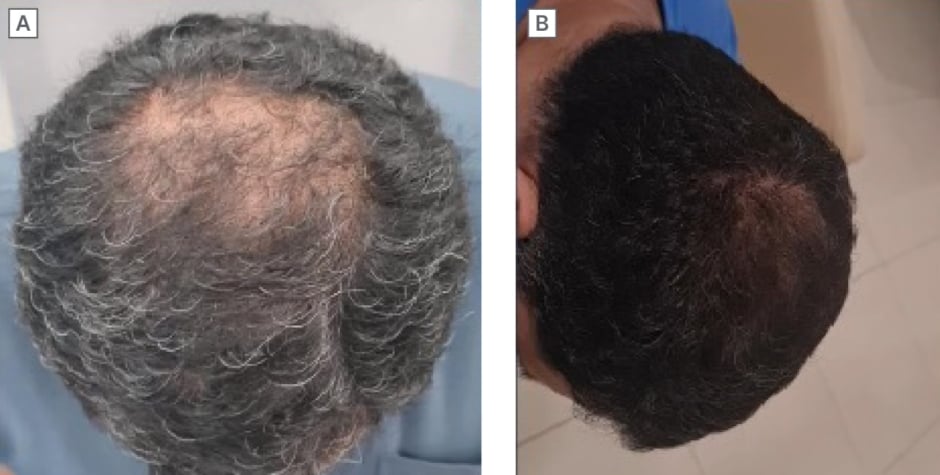
Figure 3: SAMiRNA-AR68 clinical case study.9
A) Baseline;
B) After 5 months of CosmeRNA ARI treatment.
In terms of patient feedback, both experts agreed that weekly application confers a convenience benefit for SAMiRNA when used to treat AGA and that this feature is viewed favourably by patients. For the patients with AGA currently using this product in his clinic, Park described it as an “amazing technology” and “totally different to other modalities like hormonal antiandrogen drugs or circulation-improving solutions such as minoxidil.” He also reiterated that feedback from patients on CosmeRNA ARI has been “overwhelmingly positive,” particularly among Arabic individuals who often prefer to avoid antiandrogenic drugs like finasteride or dutasteride. “For many, CosmeRNA has become the go-to alternative,” Park commented. “Patients have reported feeling safety and confidence using this product, with noticeable improvements in their hair health and regrowth. Notably, some have shared that they experienced a significant reduction in AGA symptoms and even saw improvements in their female pattern hair loss.”
“Previously, there has not been a single effective way to improve female pattern hair loss except for low-dose minoxidil medication or similar,” he explained. “These success stories therefore highlight the unique benefits that CosmeRNA offers as a non-hormonal treatment option.”
Integration Into Clinical Practice
Park acknowledged that a frequent question from HCPs is whether SAMiRNA technology can be used in conjunction with other treatments like antiandrogenic agents. “Since CosmeRNA works by reducing androgen receptors, it is indeed effective when used alongside medications that lower androgen levels. Combining CosmeRNA with these drugs can potentially enhance treatment outcomes,” he confirmed. “A common misconception among HCPs is that CosmeRNA ARI is solely an alternative to antiandrogenic drugs,” Park continued. “While CosmeRNA can be used as an alternative, it is also highly effective when used in combination with these other medications. Educating HCPs on the versatility of CosmeRNA, whether as a standalone treatment or in conjugation with other therapies, will be key to maximising the potential in clinical practice. Ongoing education and clear communication about this complementary use with antiandrogenic drugs will help dispel any myths and enhance the integration into treatment protocols.”
Vañó-Galván agreed that a key advantage of SAMiRNA is its capacity to be used both as monotherapy and in combination with other treatment approaches. “A good thing is that we can combine this mechanism of action: the inhibition of protein synthesis and the decrease of the androgen receptors,” he elaborated. “We can use it for men or women, and we can combine it with oral minoxidil, and even with low-dose oral dutasteride.”
“When we see the results of the studies, we see the effects of this treatment used in monotherapy but the good thing in real clinical practice is that we can combine several treatments as this mechanism of action is very innovative,” Vañó-Galván continued. “It is yet to be elucidated, but I guess that the combination of this mechanism of action with the classical antiandrogenic treatments like low-dose oral dutasteride will be really effective,” he suggested.
Overall, Park described the process of integrating SAMiRNA into pre-existing treatment protocols for AGA as “relatively smooth,” whether the agent is used as monotherapy or in combination with other treatments. “The feedback has been extremely positive from both HCPs and patients,” he noted. “As soon as the initial results became apparent, the popularity of SAMiRNA surged significantly. This flexibility in its application has made it a valuable addition to clinical practice, fitting seamlessly into various treatment regimens while delivering impressive outcomes.”
Discussing practical solutions to the challenges of implementing SAMiRNA, Park emphasised that support and resources have proved helpful, both in addressing clinicians’ questions and helping to facilitate use of the product in real-world practice. “Education and reassurance have been crucial in helping HCPs effectively integrate CosmeRNA into their practice. Comprehensive training and resources that cover everything from the science behind CosmeRNA to its practical application have empowered professionals to confidently recommend it to their patients,” he explained.
Ultimately, when managing AGA, “it is important for HCPs to use a holistic approach, combining nutritional support, conventional treatments, and CosmeRNA to maximise the potential for hair regrowth and overall hair health,” Park concluded.






