Abstract
Objective: Infantile colic is a frustrating impasse that affects up to 20% of infants. Even though its pathogenesis is currently unknown, some hypotheses are food hypersensitivity or allergy, gut dysmotility, inflammation, and visceral pain. The use of probiotics in treatment and prevention of infantile colic is a relatively new topic.
Method: Literature searches were conducted using Ovid MEDLINE®, EMBASE®, and the Cochrane Central Register of Controlled Trials. Randomised controlled trials including the terms “neonate(s)”, “infant(s)”, “probiotics”, “synbiotics”, “Lactobacillus”, “Bifidobacterium”, “colic”, and “prevention” were included.
Results: Three studies showed the different composition of intestinal microbiota between colicky infants and control groups. In six of the studies, probiotic and/or synbiotic supplementation significantly decreased the rate of crying and pain in colicky infants compared with placebo; however, in two studies, no effect on the incidence and frequency of colic-related restlessness was detected. In all, the reviewed studies demonstrated that probiotic and/or symbiotic treatment regimens were effective for infantile colic prevention.
Conclusions: There is much evidence suggestive of diversity in the intestinal microbiota among colicky and healthy infants. Based on recent research, using probiotics and synbiotics is a practical and favourable strategy for prevention and treatment of fussiness in colicky infants.
INTRODUCTION
Infantile colic (IC) is defined as paroxysms of crying or fussing due to abdominal pain for ≥3 hours a day, occurring 3 days or more per week for 3 weeks, in a healthy infant aged from 2 weeks to 3 months.1,2 IC’s prevalence is estimated to be up to 20% in the general population and is well-known as a frustrating problem among parents and healthcare professionals.3 The condition usually manifests at about 2 weeks of age and no longer exists by 4 months of age.
Although the pathogenesis of IC is presently unknown, it is likely due to a multitude of factors, with many theories proposed. Psychosocial theories, such as inadequate maternal–infant interactions, family tension, maternal anxiety, depression, and smoking, are potentially risk factors.4,5 Gastrointestinal hypotheses mention increased intra-abdominal gas, visceral pain, and immaturity of the nervous or digestive system.4,6
Despite there being several benign treatment modalities available, there is no gold standard treatment option for colicky infants. The current management technique recommended is the provision of support and reassurance to the parents,7-10 although, in breast-fed infants, the use of hypoallergic foods and the exclusion of cow’s milk protein from the mother’s diet has been suggested.7-9,11 In bottle-fed infants, using hypoallergenic formulas was shown to be effective in a few studies.7,8,12
Pharmaceutical treatment has limited benefit in the management of colicky pain. While agents such as dicyclomine, simethicone, and nutritional supplements can be useful in some infants, few randomised controlled trials (RCT) support their efficacy.13-15 Currently, recent evidence has suggested probiotic and synbiotic usage for the improvement of colicky pain.16,17
While the need to find a treatment for infantile colic may not appear immediately obvious because of the condition’s benign and ultimately self-resolving nature, infantile colic can increase the risk of maternal depression,18 early breastfeeding discontinuance,19 and shaken baby syndrome.20 In this context, the imperative to develop a viable therapeutic option becomes more pressing.
Ancient physicians in the Middle East used yogurt for curing disorders of the stomach and intestines.21 In the Persian variant of the Old Testament, it is stated that “Abraham owed his longevity to the consumption of sour milk” (Genesis 8:18).22
In 1908, the Russian scientist Elie Metchnikoff proposed that the long life of Bulgarian peasants came from their consumption of fermented milk products, and this theory has led to the increasing popularity of probiotics and intestinal microbiota research.21
In 2001, a joint Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) expert consultation defined probiotics as ‘live micro-organisms which, when administered in adequate amounts, confer a health benefit on the host.’23 Prebiotics are defined as ‘non-digestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, thus improving host health.’24 Synbiotics are products that contain both probiotic and prebiotic components.
Previous studies have shown the safety of probiotic and synbiotic medications in healthy children.25 In 2014, a study analysing 57 clinical trials that administered probiotics and synbiotics to infants between 0 and 24 months old found there were no major adverse effects.26 Diarrhoea, vomiting, and bloating are the most common adverse effects.27,28 Because of limited evidence, probiotic administration in high-risk groups (such as preterm infants and immune deficient children) may be contraindicated.29,30
Gut–Brain Axis and Microbiota
The intestine of a newborn infant is essentially sterile, and early postnatal life is known as the bacterial colonisation phase. The main sources of the colonising bacteria are the mother and the environment.31,32 The collection of genomes of these microbes is recognised as the human microbiome.33
The presence of these microbiota is crucial for the infant’s physiology, including the evolution of the gastrointestinal (GI) tract and health of the immune system. Recent studies have also revealed the requirement of gut microbiota for the normal functioning of the central nervous system (CNS).34,35
As mentioned, gut microbiota consist of various communities of bacteria and their structure and activity have recently been better identified through the use of molecular and metagenomics tools. Lots of bacterial phyla are present in the GI tract, such as Actinobacteria, Bacteroidetes, Firmicutes, Fusobacteria, Proteobacteria, and Verrucomicrobia,36 with Bacteroidetes and Firmicutes accounting for 70–75% of microbiomes.36,37
Genetics, prematurity, mode of delivery,10 age, diet, metabolism, geographic region, stress, and probiotic or antibiotic consumption are factors that can impact the infant microbiome.38-40 An adult human intestine contains about 100 trillion necessary bacteria,32 and by 2 years of age the child’s gut microbial profile begins to look more like that of an adult.25
Currently, many studies support the functional link between the GI tract and the CNS41; this is referred to as the gut–brain axis (GBA). This complex consists of the hypothalamic pituitary adrenal axis, CNS, the autonomic nervous system, and the enteric nervous system.42 The central nervous system and, in particular, the hypothalamic pituitary adrenal axis, can be activated in response to environmental factors, such as changes in emotion or stress. The hypothalamic pituitary adrenal axis stimulates cortisol release and is driven by a complex interaction between the amygdala, hippocampus, and hypothalamus, which constitute the limbic system. Hypothalamic secretion of the corticotropin-releasing factor stimulates adrenocorticotropic hormone secretion from the pituitary gland that, in turn, leads to cortisol release from the adrenal glands. In parallel, the central nervous system communicates along both afferent and efferent autonomic pathways, with different intestinal targets, such as the enteric nervous system, muscle layers, and gut mucosa, modulating motility, immunity, permeability, and secretion of mucus. The enteric microbiota has a bidirectional communication with these intestinal targets, modulating gastrointestinal functions and being itself modulated by brain–gut interactions.42
Thus, the gut–brain axis is associated with involvement in the pathogenesis of certain CNS disorders, such as autism spectrum disorders,43 anxiety, depression,44 and chronic pain.31
Information transport between the GI tract and the brain takes place via four major pathways:
- Vagal and spinal afferent neurons.
- Immune system signalling.
- Endocrine signalling.
- Microbial factors.43,45,46
These pathways are closely associated with each other.47
In many of these communication pathways, the important role of the biologically active gut peptides and neuropeptides are clear. Manipulation of the microbiota with antibiotics,48 probiotics, synbiotics, functional foods,49 and also faecal microbial transplantation50 and germ-free animal models,51 has shown the important role of gut microbiota–brain interactions.42
There is evidence suggestive of diversity in intestinal microbiota among colicky and healthy infants,52 with lower biodiversity in the stool microbiota of colicky infants, including a diminished number of lactobacilli and greater counts of Gram-negative bacteria.52
In this review, the authors discuss the literature on using probiotics for the treatment of infant colic, and then shift their focus to recent trials focussing on prevention of infant colic via prebiotic, probiotic, and symbiotic interventions.
METHODS
Search Strategy
Eligible studies published between January 1966 and August 2018 were identified from within the National Library of Medicine. Many databases were searched, including Ovid MEDLINE®, EMBASE®, and the Cochrane Central Register of Controlled Trials. A number of search terms were used: “neonate(s)”, “infant(s)”, “probiotics”, “synbiotics”, “lactobacillus”, “Bifidobacterium”, “colic”, and “prevention”. There was no language restriction to the search.
Study Selection
All RCT that compared probiotics to placebo or other forms of treatment in healthy infants <4 months of age were included. All definitions of infantile colic were accepted. Articles in any language were considered as long as there was an abstract in English indicating content.
Data Extraction
Reviewers assessed eligibility of retrieved articles and abstracted descriptive data on the subjects, type of intervention, outcomes, and methodological quality. Miscalculations were resolved by consensus and discussion.
Methodological Quality of the Studies
To determine the methodological quality of selected trials, the standard methods of the Cochrane Collaboration were used. For each trial, information was pursued regarding the method of randomisation, allotment concealment, blinding, and complete follow-up, as well as noted outcomes of all studied infants. The methodological details of the studies were extracted from published data.
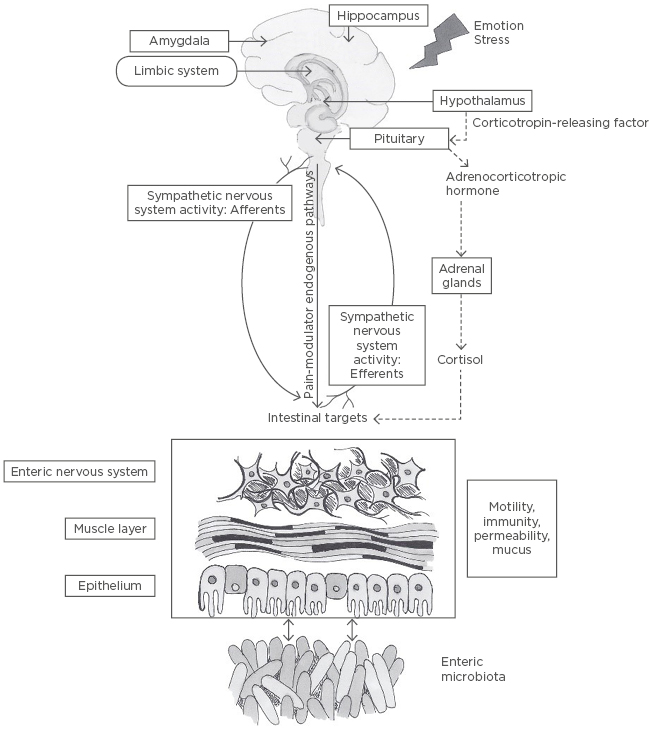
Figure 1: The role of the gut microbiome in the gut-brain axis.
The central nervous system and, in particular, the hypothalamic pituitary adrenal axis (represented by the dashed line), can be activated in response to environmental factors, such as changes in emotion or stress. The hypothalamic pituitary adrenal axis stimulates cortisol release and is driven by a complex interaction between amygdala, hippocampus, and hypothalamus, which constitute the limbic system. Hypothalamic secretion of the corticotropin-releasing factor stimulates adrenocorticotropic hormone secretion from the pituitary gland that, in turn, leads to cortisol release from the adrenal glands. In parallel, the central nervous system communicates along both afferent and efferent autonomic pathways, with different intestinal targets, such as the enteric nervous system, muscle layers, and gut mucosa, modulating motility, immunity, permeability, and secretion of mucus. The enteric microbiota has a bidirectional communication with these intestinal targets, modulating gastrointestinal functions and being itself modulated by brain–gut interactions.42
RESULTS
Probiotics for the Treatment of Infantile Colic
Lots of evidence shows the different composition of intestinal microbiota between colicky infants and control groups. In some studies, a difference in the gut microbiota has been reported.53-56 Some studies have shown an increase in the number of pathogenic bacteria and a reduction in butyrate-producing bacteria, thus promoting intestinal inflammation and pain.16,52,57
One RCT compared the microbial composition in faecal samples of colicky infants receiving L. reuteri or placebo. Roos et al.58 concluded that increasing Bacteroidetes levels in responder infants determined a decrease in colicky symptoms related to the changes of the gut microbiota.
The type, amount, duration of intervention, study population, and environmental background influence the therapeutic effects.21,59,60
In a previous study,16 the authors used a synbiotic, a mixture of a higher dose (1×109) of seven probiotics plus prebiotic fructo-oligosaccharide (FOS) with significant results.
Fifty breastfed infants aged 15–120 days with infantile colic randomly received either the synbiotic sachet (containing 1 billion colony-forming units of L. casei, L. rhamnosus, Streptococcus thermophilus, Bifidobacterium breve, L. acidophilus, B. infantis, L. bulgaricus, and FOS) or placebo daily for 30 days. Reduction in the daily crying time (>50%) was significantly higher in the synbiotic group (82.6%) compared with placebo (35.7%) at Day 7. At the end of 30 days, treatment success was 87% versus 46% in the synbiotic and the placebo group, respectively.
In 2010, Savino et al.,61 compared 46 breastfed colicky infants receiving probiotic L. reuteri with placebo. Infants of the L. reuteri-treated-group showed significantly lower crying on Day 7, 14, and 21. This study suggested that gut microbiota modification made by L. reuteri may be involved in the improvement of colicky symptoms.
Other researchers argue that exclusively or predominantly breastfed colicky infants profit from L. reuteri DSM 17938 compared with placebo.62 Saavedra et al.17 revealed that a formula containing two strains of probiotic (B. lactis and S. thermophiles) reduced the irritability of colic. A review article63 supports the positive effects of probiotic supplementation in infantile colic treatment. L. reuteri (American Type Culture Collection Strain 55730 and DSM 17938) notably decreased the rate (minutes per day) of crying and pain; additionally, no short-term side effects were identified. On the other hand, a number of trials failed to show the benefit of probiotics in colicky infants.64 In a review of three studies using synbiotic formula (consisting of B. longum and B. animalis plus a combination of galacto and FOS) in infancy, synbiotics were shown to have no effect on incidence and frequency of colic restlessness.65 Altogether, L. reuteri DSM 17938, L. casei, L. rhamnosus, L. bulgaricus, L. acidophilus, S. thermophilus, B. breve, B. infantis, and FOS are the most effective probiotic formulations being used for the treatment of infantile colic.66 The trials included in this study have been summarised in Table 1.
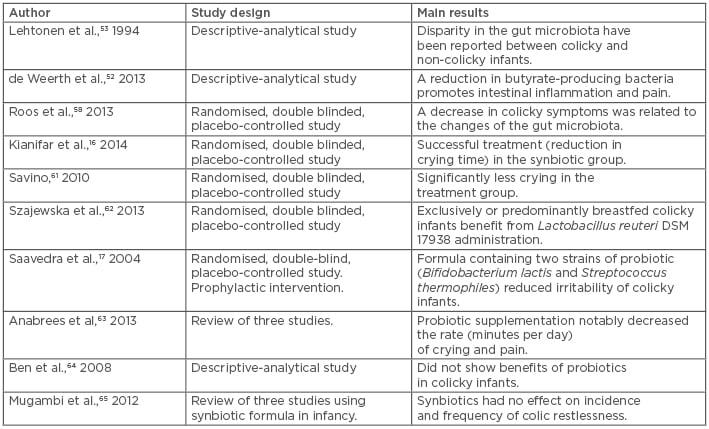
Table 1: Comparison of studies examining the use of probiotics and/or synbiotics for infantile colic.
Probiotics as a Potential Preventive Intervention for Infantile Colic
A study showed that early life probiotic administration could prevent the onset of GI functional symptoms.67 The mechanism of action of probiotics in this field has not been determined but appears to be mediated by activity on colonic intrinsic sensory neurons with an improvement in gut motility, as well as having positive effects on function and visceral pain.68 Probiotics such as Bifidobacteria showed in vitro anti-inflammatory properties and the ability to inhibit coliform growth, which has a significant presence in colicky infants, and some probiotics exert a direct action on the bacterial growth through bacteriocins production and final fermentation products, inhibiting pathogens, or feeding commensals.69
To determine whether excessive crying in infants is preventable by probiotic administration, Pärtty et al.70 randomised 94 preterm infants, breast and formula-fed, with birth weights >1,500 g and gestational ages of 32–36 weeks, in a double-blind study. From their first 3 days of life they received a mixture of galacto- oligosaccharide and polydextrose (prebiotic group), L. rhamnosus GG (probiotic group), or placebo, for 2 months. Follow-up meetings were arranged at the age of 1, 2, 4, 6, and 12 months. Compared with the placebo group, both the prebiotic and probiotic groups displayed less frequent crying (19% versus 19% versus 47%, respectively; p=0.02). The infants’ faecal microbiota investigation at 1 month of age shows that the percentage of Lactobacillus–Lactococcus–Enterococcus bacteria (14.5% versus 10.5%; p=0.005) and Clostridium histolyticum (13.2% versus 10.4%; p=0.11) was greater in excessive criers than in content infants across all three study groups.
The authors showed that early administration probiotics in infants provided relief to their crying and fussing. Based on this study, delayed cloning by B. infantis can constitute a risk factor for increasing irritability in preterm infants.70
A 2014 RCT performed in 589 breast and formula-fed infants found that the daily administration of L. reuteri DSM 17938 from Day 3 for 90 days resulted in a significant reduction in crying time (almost 51 min per day at 1 month, 33 min per day at 3 months).71 Another RCT that involved both breast and formula-fed infants showed that prophylactic use of L. reuteri DSM 17938 during the first 3 months of life reduced the magnitude of crying and functional gastrointestinal disorders.72
CONCLUSION
Probiotics supplementation, especially L. reuteri, seems to be safe and effective in the management of infantile colic-related pain and fussiness without causing notable side effects. In the deficit of other efficacious treatment modalities, their roles are valuable. Recent studies showed potential benefits of probiotics for prevention of colic. Higher quality and multicentre RCT with longer follow-up, well-defined outcome measures, and various probiotic and prebiotic mixtures are needed to identify the most effective product in the prevention and treatment of infant colic.