Meeting Summary
Adding a molecular perspective to the traditional multidisciplinary management of cancer patients is substantially hampering the adoption of precision therapy. Indeed, at this year’s European Society for Medical Oncology (ESMO) Congress in Munich, Germany, gathering >28,000 healthcare professionals spanning a range of disciplines, fields, and stakeholder groups, and >500 invited speakers, much attention focussed on discussing how to facilitate the integration of molecular data in the clinical management of cancer patients.
INTRODUCTION
A number of novel treatment options, either in the form of new agents or updated therapeutic strategies, with sequential drug exposure and dosage adjustments, were presented and debated during the event. Furthermore, along with the novelties in the drug space, the scene was equally occupied by the companion diagnostic compartment, with a substantial number of dedicated workshops, satellite events, and new product launches. Three critical factors have emerged as necessary for any meaningful molecular diagnostic approach to impact patient outcomes through clinical actionability.
Tumour Tissue Requirements
The need for minimal tissue sample starting material should be a basic requirement for any test to be broadly introduced into routine clinical practice. Low input means, for example, being able to assess genomic variants from small needle biopsy and cytological specimens. This is commonly used for non-small cell lung carcinoma (NSCLC) diagnosis and will eventually lead to more patients with actionable results.
Testing Turnaround Times
Complete biomarker results should be available within days rather than weeks. Indeed, many European institutions have started to build in-house sequencing facilities to reduce the time to obtain final results; this allows clinicians to start treating patients more quickly, aiming to achieve diagnosis and treatment initiation within days (Figure 1). Conversely, also hampered by logistical issues, the institutions that outsource tests can take weeks to deliver data,1,2 a timeframe that is no longer acceptable, especially in cases such as late-stage NSCLC. In addition, when the number of samples to be tested reaches substantial proportions, the outsourcing testing strategy can result in increased pressure on the healthcare system due to third-party margins along with shipment fees, leading to higher overall costs regardless of the payer.3
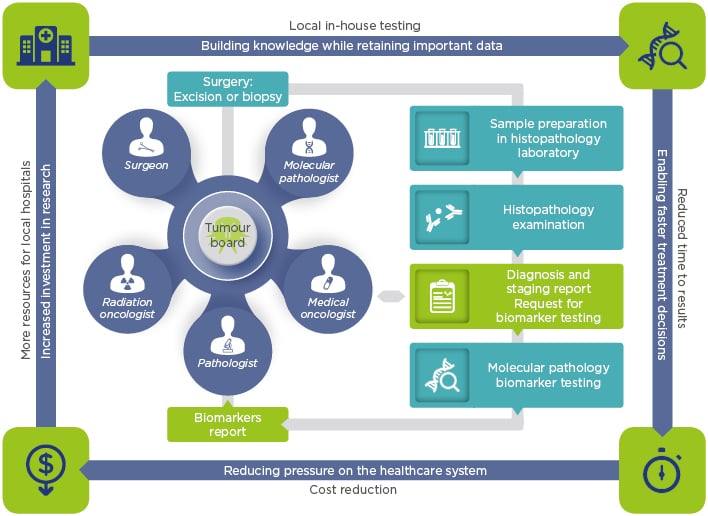
Figure 1: In-house testing and its associated benefits.
A model to fit molecular tumour boards and foster local interactions among healthcare professionals, generating and retaining both knowledge and precious data.
Adequate Biomarker Coverage
Testing should include updated relevant biomarkers based on current knowledge and ongoing late-phase clinical trials. In fact, while offering a single, very large panel testing for hundreds of genes, of which many currently hold limited or no clinical actionability, biomarkers covering a plethora of genomic variants for many cancer types might be a very attractive research-oriented solution; a dedicated test covering clinically relevant genes is a more pragmatic and cost-effective approach. Furthermore, in 46–80% of patient cases, the starting material is too minimal for large panel analysis.2,4 In some cases, this testing approach may require a rebiopsy, which comes with associated risks, elevated costs, and treatment delays or, when not applicable, can lead to suboptimal therapy selection.
While in the early years of biomarker testing outsourcing was a logical choice due to technical and investment constraints, nowadays outsourcing is largely reduced due to the fast development of sequencing technologies and the dramatic reduction in the cost of in-house biomarker testing. Therefore, sending to third-party labs is now a non-sustainable long-term approach.
Finally, given that precision oncology is a medical practice deployed at the local level, at the patient’s bedside, and mostly via interactions between local healthcare professionals, in-house molecular profiling is the best fit to this model. In many of the ESMO-hosted discussions, it was clear that the flexibility to triage patient samples and to discuss in depth the findings at local tumour boards is key to providing optimal, truly personalised care.
NOVEL OPPORTUNITIES FOR LUNG TUMOUR TREATMENT AND TESTING
Targeted agents, such as the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors gefitinib, erlotinib, afatinib, and osimertinib, have considerably transformed the management of patients with EGFR-mutated NSCLC, representing one of the most significant advances in lung tumour treatment for decades. The introduction of these agents into clinical practice has been developed along with the advances made in the molecular pathology field and the broad adoption of next-generation sequencing (NGS), allowing a robust and sensitive evaluation of EGFR status.
Furthermore, the recent success of immunotherapy in the metastatic NSCLC setting (independent from the EGFR status) has revolutionised the entire treatment scenario. Nonetheless, >50% of treated patients show no clear evidence of responding to immune checkpoint-blocking (ICB) therapies, underlining the need to develop new, robust predictive biomarkers that should appropriately guide the selection of ICB agents.5 Programmed death-ligand 1 (PD-L1) expression, assessed by immunohistochemistry (IHC), has emerged as a predictive marker for the use of ICB agents in NSCLC.6 However, despite currently being the most commonly used biomarker in the immune oncology space, the analysis of PD-L1 by IHC comes with several significant challenges, including variability in preanalytical conditions, the use of different antibodies along with different staining platforms, the lack of an unequivocal type of scoring system, the typical IHC interobserver variability, and the issue of intrinsic tumour heterogeneity.5,6 Additionally, it is currently acknowledged that some patients with low or no PD-L1 expression may still benefit from checkpoint inhibition. Recently, the tumour cell mutational burden (TMB) status has been correlated with distinct degrees of clinical benefits in ICB-treated patients with various tumour types, including NSCLC.6 High mutational load/burden is commonly defined as ≥100 non-synonymous single nucleotide variants per genome as identified via whole-exome sequencing;7 however, this threshold can greatly vary between tumour types.7 Thus, major challenges remain to improve the robustness of TMB and eventually introduce it into routine diagnostics. Among these challenges, the definition of the optimal tumour purity, the minimal sequencing depth, and the need to identify an appropriate threshold for defining high and low mutational burden for different tumour types are key.5,7 In summary, while PD-L1 expression and TMB value may aid the identification of ICB responders, they identify distinct subclasses of patients and are not a clear dichotomous set of biomarkers.8-10 Outcomes from ESMO 2018 highlighted the need to further expand our understanding behind response to ICB, for example, focussing on the activity of tumour-infiltrating T cells through T cell receptor characterisation via sequencing.
Hampered by compelling evidence in the metastatic setting, accumulating data from preclinical investigations and retrospective studies of human lung cancer samples have been discussed, suggesting the presence of an immunosuppressive microenvironment in the early stage of the disease. This serves as another indicator of the importance of investigating the role of T cells via repertoire analysis and an important reason to thoroughly investigate the role of T cells and T cell receptors. In fact, immunotherapy is now also studied in non-metastatic NSCLC.11 Trials of checkpoint inhibitors have recently been completed (or are currently ongoing) in early-stage resectable NSCLC in different settings (neoadjuvant, combined neoadjuvant, and adjuvant therapy) and in various combinations with standard of care modalities. For example, very encouraging results have been reported in the PACIFIC trial,12 wherein progression-free survival (PFS) was significantly longer with durvalumab versus placebo after chemoradiotherapy in Stage III NSCLC. This is an important development for immunotherapy, given that 30–60% of patients with Stage I–III NSCLC will ultimately develop post-resection metastases.11
Another important option for lung cancer treatment, largely discussed at this year’s ESMO Congress, relies on agents targeting genomic fusion products. For instance, ALK and ROS1 rearrangements define an important molecular subgroup (3–5% of cases) in advanced NSCLC, with major clinical implications. Now that alectinib has replaced the first-in-class ALK/ROS1/MET inhibitor (crizotinib) as the standard first-line therapy for ALK-positive advanced NSCLC,13 it is becoming clear that, after initial response to treatment, resistance develops and patients invariably progress. While other potent ALK inhibitors and brain-penetrable compounds have been approved, including brigatinib, ceritinib, and lorlatinib, questions remain concerning the optimisation of treatment sequencing strategies to prevent or reduce resistance. To this end, performing highly sensitive molecular profiling, allowing the detection of new rising genomic alterations and eventually impairing clinical response, will support the development of these novel treatment schemes.
Furthermore, tyrosine receptor kinase (TRK) fusion agents took to the stage at this year’s ESMO Congress. A genomic rearrangement known as TRK fusion occurs when a member of the neurotrophic tyrosine receptor kinase (NTRK) gene family fuses with another unrelated gene, producing an altered tropomyosin receptor kinase (Trk) protein.14 This novel protein product is permanently activated (i.e., uncontrolled kinase function), triggering a constant oncogenic signal cascade, which becomes the primary driver of tumour cell growth in patients with TRK fusion-positive cancer. NTRK1/2/3 gene fusions occur in various adult and paediatric solid tumours with varying prevalence, including appendiceal cancer, cholangiocarcinoma, colorectal cancer, gastrointestinal stromal tumours, infantile fibrosarcoma, lung cancer, mammary analogue secretory carcinoma of the salivary gland, melanoma, pancreatic cancer, thyroid cancer, and various sarcomas.15 Only sensitive and specific tests can reliably detect TRK fusion-positive events. The ESMO Precision Medicine Working Group has released specific suggestions16 that recommend RNA-based NGS testing as the preferred method to investigate genomic alterations such as gene fusions. Fluorescence in situ hybridisation can also be used to test for TRK fusion cancer, while IHC can detect the presence of the Trk protein. However, both approaches substantially lack sensitivity and specificity, thus leading to suboptimal patient selection.
Among the presented new agents, entrectinib is a central nervous system-active potent inhibitor of all Trk proteins, as well as ROS1 and ALK.17 Prof Demetri, Boston, Massachusetts, USA, presented an integrated efficacy and safety analysis from three Phase I/II clinical trials using entrectinib: ALKA,18 STARTRK-1,19 and STARTRK-2.20 Data show that treatment with entrectinib induced responses that were durable in >50% of treated patients. Notably, entrectinib is well tolerated with limited side effects and induces clinically meaningful systemic responses across tumours with a variety of histologies and in patients with and without central nervous system disease. This represents an advance in precision medicine, with entrectinib offering benefits for NTRK– fusion-positive patients as a tumour agnostic targeted therapy. Based on these results, screening patients for NTRK gene fusions in solid tumours should be actively considered.
NEW OPPORTUNITIES IN OVARIAN AND BREAST CANCERS: THE NEED FOR BRCA TESTING
During the ESMO Congress, the first Phase III study of a poly (ADP-ribose) polymerase inhibitor as maintenance therapy after first-line chemotherapy for ovarian cancer positive findings were reported. In the SOLO1 trial21 of olaparib in patients with BRCA-mutated advanced ovarian cancer, the primary endpoint (investigator-assessed PFS) was successfully met, with a statistically significant and clinically meaningful improvement compared to placebo. At a median follow-up of 41 months, maintenance olaparib reduced the risk of disease progression or death by 70% compared to placebo. These unmatched findings were reinforced by a significant improvement in median time to first subsequent therapy or death (51.8 months for olaparib versus 15.1 months for placebo). Notably, adverse events were mostly low grade, while health-related quality of life scores did not change from baseline following olaparib exposure. These data suggest that poly (ADP-ribose) polymerase inhibitors may have an earlier entry point in the treatment of ovarian cancer and underline the importance of determining BRCA status at diagnosis by sequencing. Dr Curigliano, Milan, Italy, commented on SOLAR-1 trial data. SOLAR-122 demonstrated a significant PFS benefit with the phosphatidylinositol-3-kinase (PI3K) inhibitor, alpelisib, plus hormone therapy, fulvestrant, compared with placebo plus fulvestrant in patients with PI3K-mutated cancer. This study also highlighted the value of determining yet another genomic biomarker status (i.e., PI3K mutations) at diagnosis by sequencing to accurately select the treating agent. Finally, robust data from a large patient population study indicate for the first time that immunotherapy could be an effective first-line option for patients with metastatic triple-negative breast cancer. Additional studies will now be conducted to reinforce these preliminary findings.
LIQUID BIOPSY FOR ROUTINE TESTING: HYPES AND HOPES
Supported by the aforementioned examples and with the advent of targeted therapies, molecular profiling is often needed to guide therapeutic decisions, both at diagnosis and following the development of resistance, resulting in multiple tissue biopsies during the disease course. However, intratumour heterogeneity and clonal evolution due to prior lines of therapies further foster the complexity of the treatment decision, representing a truly challenging task for current therapeutic approaches.23 Dr Besse, Villejuif, France, in multiple appearances during the ESMO Congress, emphasised that while the gold standard method for molecular profiling involves the examination of DNA/RNA extracted from a tissue biopsy, some clear drawbacks are associated with this approach. For instance, the lack of feasibility in some cases due to the anatomical position of the tumour mass, the invasiveness of the procedure, and the possible acquisition of insufficient tissue all lead to suboptimal overall testing quality of gene sequencing.24 In the oncology community, interest is growing in the use of less invasive and costly approaches, such as liquid biopsy, analysing circulating tumour DNA (ctDNA) released into plasma from cancer cells during apoptosis or necrosis.25
Nowadays, ctDNA tests are used primarily for patients when tissue samples are not available, or to guide targeted therapy in specific clinical situations (e.g., resistance after tyrosine kinase inhibitor treatment). Dr Dienstmann, Barcelona, Spain, commented that during a study of patients receiving osimertinib, MET amplification (15%) and EGFR Cys797Ser mutation (7%) were the most common resistance mechanisms, with no evidence of an acquired EGFR Thr790Met mutation. Conversely, the incidence of Thr790Met mutation in the standard-of-care arm was found in about 47% of cases. These findings underline the need to sequence ctDNA with a multiplex gene panel-based approach rather than with a single gene testing approach (i.e., only looking for Thr790Met).26
In addition, much hope is pinned on the further development of liquid biopsy applications, for example, detecting minimal residual disease or risk of relapse in early stages, a path that is actively explored in several ongoing studies.27 For example, Dr Bonanno, Padova, Italy, presented interim findings from the MAGIC-1 trial28 suggesting that changes in plasma levels of a KRAS mutation significantly correlated with the radiological assessment of disease progression in patients with advanced NSCLC receiving either chemotherapy or immunotherapy. Moreover, the data also suggest that, in patients receiving immunotherapy, early reduction of the mutated allele abundancy in plasma may predict favourable outcomes.
CALLING ON MOLECULAR DIAGNOSTICS
Given the rapidly increasing amount of genomic information available, thanks to the reduction in sequencing cost and the democratisation of molecular profiling, clinicians are now confronted with the need to prioritise driver over passenger genomic alterations and choose the most appropriate treatment when multiple targetable alterations are found.
During the ESMO Congress 2018, key opinion leaders from across the globe highlighted that molecular tumour boards, a new form of interaction for medical professionals, are rapidly diffusing into major cancer reference centres.23 The main goal for a molecular tumour board is to match the unique genetic profile of a patient’s cancer with a drug (or combination therapy) having the highest evidence of clinical actionability, or to explore the possibility of enrolling patients into a recruiting clinical trial. Dr Curioni, Zurich, Switzerland, part of the ESMO press committee, highlighted that lung cancer is an extraordinary example that demonstrates the value of multidisciplinary discussion of molecular tumour profiling data, which has resulted in a dramatic improvement in the prognosis of cancer patients harbouring driver genetic alterations in genes like ALK, ROS1, EGFR, cMET, BRAF, and NTRK.29 Prof André, Villejuif, France, highlighted the first ESMO scale to rank and prioritise genomic alterations: ESMO scale for clinical actionability of molecular targets (ESCAT). ESCAT aims to improve the interpretation of sequencing results and link them to appropriate clinical trials. Furthermore, the ESMO Precision Medicine Working Group released updated recommendations concerning sequencing practice, including detection of TRK fusions, microsatellite instability, and a general guide on how to handle genetic variants detected by NGS. Also highlighted was the unmet need to integrate multiple layers of data from curated available sources (National Cancer Institute [NCI], The Cancer Genome Atlas [TCGA], ESMO-OncologyPro, and ESCAT) and present them in an intuitive and accessible manner while still accurately offering a complete picture of an individual’s medical profile in the form of a clinical decision support tool. This represents the next challenge for companies serving cancer-treating clinicians. The key to success for such a tool is a simple and direct workflow of guided steps for clinicians to navigate the magnitude of molecular information, enabling decisions to be made as quickly and reliably as possible. Moving in this direction, the ESMO Magnitude of Clinical Benefits scale (ESMO-MCBS) is a tool designed to assess the clinical benefit of different cancer medications, allowing stakeholders to discriminate between high-value treatments (i.e., improving survival and/or quality of life of cancer patients, from modest to marginal approaches). At this year’s ESMO Congress, a set of workshops aimed to address how to prepare the next generation of the ESMO-MCBS for the integration of molecular diagnostics-related benefits in the cost calculation process.30 Given that diagnostics-related reimbursement policies across the European Union (EU), the USA, and the Asia-Pacific region will likely evolve in the near future, much attention will be paid to this topic at next year’s Congress.
Overall, the ESMO Congress highlighted that in order to make precision medicine the global standard of care, including the wide application of genome-analysis in the form of a feasible diagnostic solution, and not only as a privileged option for a few national healthcare systems, a variety of socioeconomic factors will need to be considered.31 Health policy makers, medical institutions, manufacturers, clinicians, and biomedical researchers, along with patient associations, will have to engage in this process through global initiatives, while being able to deploy them at the local level.32
CONCLUSION
To conclude, the list of new therapies associated with specific biomarkers is growing steadily. What was once a vision for improved cancer care is now a reality, with molecular insights resulting in better patient management, reduced treatment side effects, and enhanced quality of life. At this year’s ESMO Congress, most key opinion leaders clearly pointed out that investments in high-quality molecular profiling for tumour patients will undoubtedly have an impact on appropriate treatment decisions and, thus, eventually impact clinical outcomes.








