Abstract
Allergic rhinitis is one of the most common chronic inflammatory conditions, affecting up to 30% of people in Europe. Allergen immunotherapy (AIT) is the only treatment for allergic rhinitis and asthma that has a disease-modifying effect, and it is recommended in European guidelines for use in conjunction with patient education, specific allergen avoidance, and symptomatic pharmacotherapy. Reported AIT adherence rates vary widely but are often low in real-world settings. Factors known to affect adherence are patient, treatment, or physician-related, and vary between healthcare settings. Misconceptions or a lack of AIT knowledge among patients with regard to efficacy and side effects may contribute to high rates of discontinuation observed during the first year of AIT treatment. Interventions to improve patient adherence are multifaceted and should focus on patient education, particularly the provision of accurate information regarding adverse effects of AIT and when to expect an improvement in symptoms, patient-support programmes, and the use of regular eHealth reminders via a telephone call, text message, or social media. Serum-based biomarkers also have the potential to play a role in evaluating early response to AIT and in monitoring treatment adherence in clinical practice. In this review, the authors explore barriers to continuation with AIT and discuss initiatives to motivate and support patients through the challenging early months of treatment, prior to the onset of clinical effect and when side effects are most common, to encourage long-term adherence to therapy and achieve optimal patient outcomes.
INTRODUCTION
Allergic rhinitis (AR) is characterised by multiple symptoms involving the upper airways, nose, and eyes.1 It is one of the most common chronic inflammatory conditions, affecting approximately 20–40% of the global population and 15–30% of people in Europe,2-6 and frequently coexists with asthma.7 AR can impair sleep and mood, negatively impacting quality of life (QoL), daily activities, social functioning, productivity, and work performance, and represents a considerable burden on public health.3,8
Allergen immunotherapy (AIT) is the only treatment for AR and asthma that has a disease-modifying effect.1 Guidelines recommend that the management of AR combines AIT with patient education, specific allergen avoidance, and symptomatic pharmacotherapy.9 AIT involves the repeated administration of allergens over a period of years (optimally 3–5 years)1,10 to reduce clinical and immunological responses to the allergens and induce allergen-specific immunological tolerance.11-14 AIT is typically administered either as monthly injections of subcutaneous immunotherapy (SCIT) or daily oral doses of sublingual immunotherapy (SLIT) as tablets or liquid drops. SCIT requires visits to the clinic for dosing, while SLIT can be administered easily at home following the initial dose.13,14
EFFICACY AND SAFETY OF ALLERGEN IMMUNOTHERAPY
AIT has proven long-term efficacy in reducing allergic symptoms and the need for symptomatic medication,1,11,12,14-18 and has also been shown to reduce the risk of progression from AR to the development of asthma symptoms and/or the use of asthma medication in children.19,20 It is important to note that an improvement, not complete resolution, of symptoms should be expected with AIT.1 The manufacturers of all AIT products within Europe must follow the European Medicines Agency (EMA) guidance for the manufacturing and quality control of allergen products of biological origin, which provides an assurance of quality and effectiveness.1,14,18
Large clinical trials have demonstrated significant improvements to the QoL of patients with grass pollen AR treated with SLIT or SCIT.10,22-25 However, there remains a need for further real-world studies examining QoL in patients receiving AIT. Both SCIT and SLIT demonstrate favourable safety and tolerability when administered in an appropriate setting and according to current guidance.1,14,15
COST-EFFECTIVENESS OF ALLERGEN IMMUNOTHERAPY
A systematic review of 24 studies conducted in Europe or North America, encompassing a range of perennial and seasonal allergic conditions due to house dust mite, grass or ragweed pollen, or a mixture of various allergens, demonstrated that AIT was associated with cost savings relative to symptomatic treatment.26 Of the 6 studies that compared SLIT with SCIT, 4 found cost savings for SLIT and 2 for SCIT.26 A health-technology assessment conducted in the UK that examined the relative costs of SLIT and SCIT showed that, when compared with symptomatic treatment, both therapies may become cost-effective at a threshold of £20,000–30,000 (equivalent to approximately €24,000–36,000 at the time of study publication in December 2013) per quality-adjusted life-year approximately 6 years after treatment initiation, or 5 years for SCIT compared with SLIT.27 However, these estimates were based on limited data and a number of assumptions.27 Since patients self-administer SLIT at home after the first dose, there is the potential for considerable time savings for both the patient and healthcare provider, thereby improving cost-effectiveness compared with SCIT.14 An economic evaluation of patients with AR found that when both direct and indirect costs were considered, 3-year expenditures per patient were €684 and €1,004 for SLIT and SCIT, respectively.28
CONTINUATION RATES WITH ALLERGEN IMMUNOTHERAPY
Adherence can be divided into the following stages: 1) initiation (also referred to as acceptance), 2) implementation (also referred to as compliance), and 3) persistence.29 Lack of adherence to treatment is a growing global problem associated with the management of chronic diseases such as AR, contributing to decreased treatment efficacy as well as potential increases in rates of hospitalisation, morbidity, and mortality.30 In general, the causes of poor adherence may be related to the patient, disease, treatment, or healthcare system.31
There is no consensus regarding an acceptable adherence rate to AIT. However, a rate >80% is generally considered to be adequate.30 Persistence and adherence to AIT is reported to be lower in real-world settings than in clinical studies.32,33 In clinical trials with a follow-up duration of up to 3 years, average adherence is around 80–90% in both adults and children.34 In contrast, adherence to AIT in real-world studies is typically poor, although there is a wide variation in reported rates; in studies of SCIT with a duration of follow-up of 3 years, adherence rates are in the ranges of 23–88% in adults and 16–89% in children.30 In SLIT studies with a similar duration of follow-up, adherence rates are in the range of 7–85% in both adults and children.30 The wide variation in adherence to AIT is similar to that observed for medication use in other chronic conditions, including rheumatoid arthritis, diabetes, chronic obstructive pulmonary disease, and cardiovascular disease.30 Possible reasons for this heterogeneity are that studies were performed in different populations and countries, and patients were treated with different allergen vaccines and treatment schedules, using different measures of adherence.30 Of particular note, research suggests that differences in rates of adherence may exist between the type of treatment regimen (i.e., SLIT versus SCIT) and certain patient groups (i.e., younger versus older patients) in the real-world setting (Table 1).
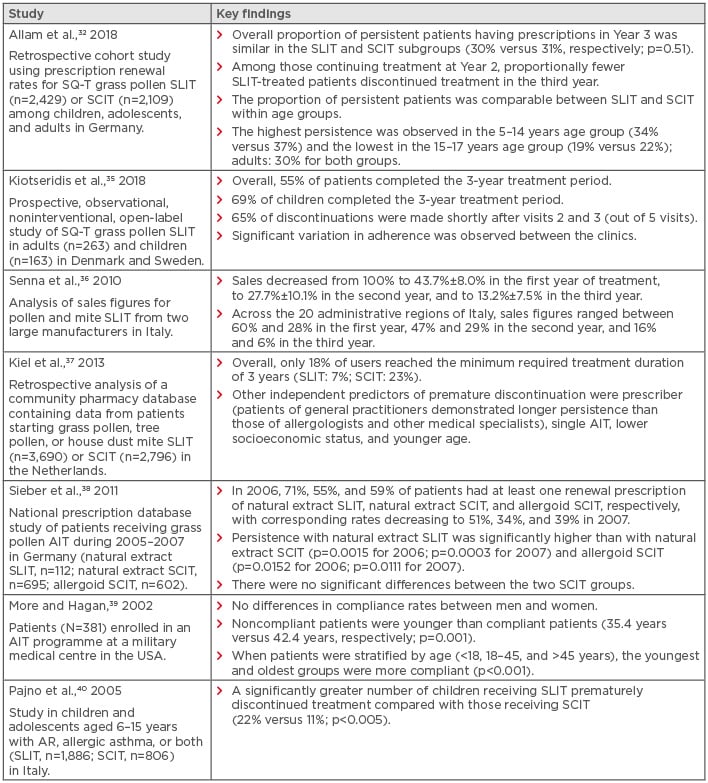
Table 1: Differences in adherence and persistence rates with allergen immunotherapy in selected real-world studies.
AIT: allergen immunotherapy; AR: allergic rhinitis; SCIT: subcutaneous immunotherapy; SLIT: sublingual immunotherapy; SQ-T: standardised-quality unit tablet.
FACTORS AFFECTING ADHERENCE TO ALLERGEN IMMUNOTHERAPY
Factors known to influence adherence to AIT include the patient’s perception of their disease severity, mode of AIT administration or regimen (i.e., sublingual versus injection and the frequency of administration), inconvenience, fear of injections, cost, treatment benefit (or lack thereof, and as perceived by both patients and physicians), side effects, and the patient’s level of knowledge about their condition and treatment.41-43 Broader factors determining adherence include a holistic approach to treatment, the physician–patient relationship, and the quality of treatment delivery.44
The first year of therapy is pivotal for AIT adherence, with evidence demonstrating that patients who adhere to their AIT schedule during this time are more likely to complete the rest of their treatment.45 A retrospective cohort study using prescription renewal rates to compare SLIT-tablet versus SCIT in patients with grass pollen allergy found that despite similar persistence over 3 years, discontinuations in the first year of treatment were more frequent among the SLIT-tablet patients.31 Those continuing treatment after the first year were less likely to discontinue treatment during the third year, compared with those receiving SCIT.32 The dose of AIT does not appear to play a notable role in adherence to therapy.31
Research has shown that greater perceived disease severity is associated with better adherence to medication.46 This is of particular relevance for patients with less severe AR, who may not experience a significantly detrimental impact on their QoL prior to starting treatment and who may therefore be less motivated to continue with therapy. Patients’ perceptions and expectations of AIT are largely dependent on their knowledge of allergic disease and the treatment options available.43 It has been suggested that there are numerous misconceptions and a lack of knowledge about AIT among patients.47 A questionnaire-based study in patients with AR or asthma found that patients often had a negative view of AIT before starting therapy.48 In a multinational, observational, internet-based survey of patients from five countries, almost two-fifths of early AIT discontinuers (i.e., those who stopped treatment before the end of the recommended course) reported a perception of poor effectiveness.41 Although AIT starts to relieve symptoms within a few weeks or months, patients may have unrealistic expectations about the speed of improvement in their symptoms when compared to their experiences with antihistamines and nasal corticosteroids.41 A questionnaire-based study found that one-fifth of participants with AR receiving AIT did not know when an improvement in symptoms should be expected after starting treatment, and almost one-fifth (18%) believed an improvement would occur within days or weeks.47 In the same study, around one-third of the study population were aware that AIT may have some potential risk or adverse effects.47 Moreover, for seasonal allergens (e.g., to plant pollens), AIT is administered perennially or started a few months prior to the pollen season,49 outside of which, patients may largely be symptom-free. If patients then experience symptoms with the onset of the pollen season (which may itself also be unpredictable and affected by weather patterns and varying daily environmental pollen loads), they may discontinue treatment due to a perceived lack or loss of effectiveness.
Adverse events (AE) are the most common reason for discontinuation of SLIT.35 AE (mostly local reactions that occur early in treatment) account for at least one-quarter of all dropouts in clinical trials and the rate is likely to be even higher in a real-life setting.14
The method of payment is a key determinant of uptake and continuation with AIT. Clear differences are evident when comparing sales of SLIT between reimbursed settings versus the patient paying out of their own pocket.14 In a study of patients with AR who discontinued SCIT in the USA, 40% reported that this was due to issues of cost, specifically inadequate or nonexistent insurance coverage.50
The type of prescriber and frequency of office visits may be predictors of continuation of AIT. A study in the Netherlands found that patients of general practitioners demonstrated longer persistence than those of allergologists and other medical specialists,37 while evidence has also demonstrated an association between more frequent physician’s office visits (such as that required for SCIT administration) and better persistence.32
Patient QoL is also thought to influence adherence.44 A retrospective, noninterventional, cross-sectional study in paediatric patients treated with SLIT showed that QoL scores, assessed using the generic 12-Item Short-Form Health Survey (mental and physical components), were comparable to those of the general population, in contrast to untreated allergic patients who typically report lower QoL, and were correlated with good adherence to SLIT.44
However, evidence from placebo-controlled trials of SLIT has demonstrated that adherence depends less on the patient’s perception of therapeutic efficacy and more on their motivation to participate in the trial and to meet the researcher’s expectations.33 It was suggested that enrolment of patients into a trial is similar to a concordance process, in which a therapeutic alliance is established between the patient and physician, and this is known to be an important factor in maintaining adherence.33 Patients also tend to be more adherent when their behaviour is being recorded or observed, a phenomenon known as the Hawthorne effect.32
INTERVENTIONS TO IMPROVE ADHERENCE TO ALLERGEN IMMUNOTHERAPY
There is a clear need to improve rates of real-world adherence to AIT14 and, although research into interventions to improve adherence to AIT is lacking, lessons learned from interventions used in other chronic health conditions may be valuable. The chronic care and patient-centred care models are two well-established models of care that can be used to improve adherence.51 The latter focusses on the importance of understanding and targeting modifiable barriers to adherence, such as patients’ knowledge and health beliefs and healthcare providers’ communication skills.51 Potential barriers to adherence and interventions that may encourage continuation with therapy are summarised in Table 2.
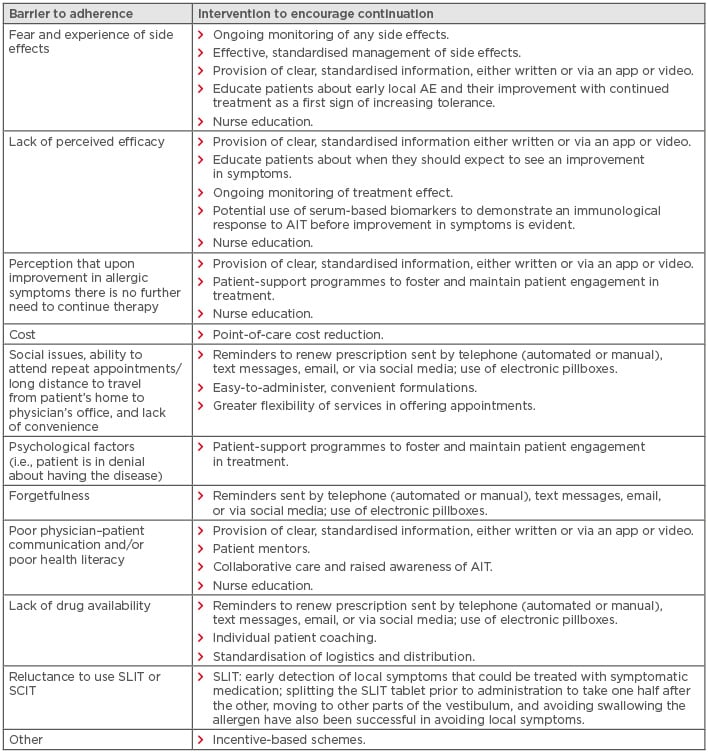
Table 2: Barriers to adherence to allergen immunotherapy and interventions to encourage continuation with therapy.11,18,29,32,35,52,53
AE: adverse event; AIT: allergen immunotherapy; SCIT: subcutaneous immunotherapy; SLIT: sublingual immunotherapy.
Adapted from Demoly et al.29
It is important to note that the factors contributing to poor adherence in one setting or healthcare system may not be applicable to other settings or systems. Ideally, immunotherapy practitioners should audit their own practice to determine the major factors affecting adherence and include these in decision-making processes regarding AIT for individual patients.
A retrospective analysis conducted in patients with AR with or without asthma found that patient education regarding the treatment course and slow effect of AIT, as well as the need for close follow-up to effectively prevent and treat adverse reactions, are all important in improving adherence to therapy.54 Improving the information given to patients by prescribers is an important first step in encouraging long-term adherence.14 An observational, open-label study showed that patients’ satisfaction with SLIT in terms of their assessment of effectiveness, tolerability, and convenience is strongly influenced by the information supplied by their healthcare provider.55 The guidelines on AIT by the German, Austrian, and Swiss allergy societies include a treatment information sheet to inform the patient about practical aspects of AIT, such as expected effects, type and duration of treatment, possible side effects, and alternative treatments.18 The SLIT information sheet can be found here and the SCIT information sheet here. Regular monitoring is associated with increased adherence and a clear relationship can be seen between number of follow-up visits and adherence to treatment;56 this is particularly important because SLIT is administered at home without direct supervision.14 The high levels of adherence found in clinical trials are thought to result partly from regular monitoring of enrolled patients.41
Evidence suggests that multifaceted patient-support programmes that encompass communication, educational, and motivational components are associated with improved adherence to AIT.29 A small study (N=52) reported a higher rate of adherence in patients who underwent educational training versus those who received only instructions about SLIT administration (96% versus 77%, respectively). Patients who did not receive additional education were more likely to discontinue treatment due to minor side effects, such as oral and gastrointestinal local reactions.57 A study to evaluate an action plan consisting of education, frequent contact, and strictly scheduled visits for patients taking SLIT found that after 1 year, 12% of patients discontinued treatment compared with 35% in the control group (p<0.001).58 In patients with chronic metabolic diseases, empowerment-based self-management interventions have stronger, long-lasting effects than conventional self-management or education.29
Forgetfulness is an issue impacting adherence, which can be accentuated by a number of patient health and lifestyle-related factors, such as age, comorbidities, travel, and social activities.29 eHealth interventions, such as the use of social media, email, and phone services to provide reminders and ongoing monitoring, are considered valuable in improving adherence to SLIT,59 particularly because these are required more frequently for daily at-home SLIT administration compared with less-frequent administration of SCIT.29 Incentive-based schemes have been shown to successfully promote medication adherence in selected clinical trials; however, the development of sustainable and cost-effective long-term interventions in the real-world setting may be a challenge.53
Until now, the efficacy of AIT has been assessed subjectively based on individual perception. The use of serological biomarkers to evaluate immunological response to AIT, even before the onset of symptom relief, may be a very helpful tool in encouraging adherence. If a lack of response is detected at an early stage, selection of the AIT allergen can then be re-evaluated. Some studies have shown that an elevated ratio of specific IgE to total IgE is a potential positive predictive marker for AIT.52 The role of serum-based biomarkers in monitoring adherence and compliance with AIT also warrants further exploration. A recent European Academy of Allergy and Clinical Immunology (EAACI) position paper recommended further research into the use of serum IgG4 as a biomarker for compliance, based on the increase in IgG4 levels observed with AIT.52
Some interventions to improve adherence have demonstrated limited success or have potential limitations. A systematic review found that <50% of the interventions used in randomised clinical trials were associated with improved adherence, and almost all of those with long-term efficacy were complex and multifactorial. Furthermore, it was not possible to predict which interventions would be successful in a particular setting and over a given timeframe.60
CONCLUSION
AIT is the only treatment for AR and asthma that has a disease-modifying effect. However, reported adherence rates with AIT are often low in real-world settings, with high rates of discontinuation during the first year of treatment. Factors known to affect adherence may be related to the patient (e.g., forgetfulness or fear of injections), treatment (e.g., lack of perceived efficacy or lack of knowledge about side effects), or physician (e.g., lack of communication or inadequate counselling), although these may vary according to the healthcare setting or system. Interventions to improve patient adherence should focus on standardised patient education, particularly regarding adverse effects and timing of improvement in symptoms, patient-support programmes, and mentoring, and the use of eHealth reminders. Providing this information via an app or video, if possible, may also promote patient engagement. Effective, standardised management of side effects and regular follow-up visits may also improve patient adherence. The potential role of biomarkers in evaluating early response to AIT and in monitoring treatment adherence in clinical practice also warrants further investigation.






