Meeting Summary
During a symposium at the 8th International Myology Congress in Paris, France, key opinion leaders discussed the need to address the balance between exercise and contraction-induced muscle injury in Duchenne muscular dystrophy (Duchenne) and Becker muscular dystrophy (Becker). They explained the significance of dystrophin in enabling healthy muscle repair, the detrimental impact of contraction-induced muscle injury in the absence of dystrophin, and the safety and potential benefits of exercise in these patients. Sharing data challenging the notion that individuals with Becker and Duchenne are “untrainable,” they emphasised the importance of tailored exercise regimens in these patients. The symposium also provided an overview of the ongoing trials of sevasemten, an investigational drug which is not approved in any territory. Unpublished 24-month data suggest it can preserve function in Becker, giving it potential as a useful adjuvant in disease management.
INTRODUCTION
The rare diseases Duchenne and Becker are X-linked recessive neuromuscular disorders.1 They are characterised by progressive, irreversible weakness and atrophy of the skeletal muscles and of the heart.1,2 With an incidence of between one in every 3,500–5,000 male births, Duchenne is the more common phenotype. It is also the more severe. Diagnosis tends to occur before the age of 5 years, and boys are usually dependent on a wheelchair by age 13.1 Life expectancy is 20–30 years, with typical causes of mortality being respiratory or heart failure.1,2 Becker, which affects between 0.1–1.8 per 10,000 males,3 is also a debilitating and degenerative neuromuscular disorder, usually developing around the age of 12 years.2 Functional decline can begin at any age, while ambulation tends to be preserved until the male’s 30s; once that muscle loss occurs, the decline in function is irreversible and continues throughout the individuals life.
Duchenne and Becker are caused by mutations in the Dystrophin gene.1 Containing 79 exons, this is the largest human gene, and is responsible for producing dystrophin,1 the central protein of the dystrophin-glycoprotein complex in skeletal and heart muscle cells.4 Dystrophin connects the actin cytoskeleton to the extracellular matrix, providing stability to the sarcolemma, as well as facilitating mechanotransduction.4,5
Joanne Donovan, chief medical officer at Edgewise Therapeutics, Boulder, Colorado, USA, explained that dystrophin plays a critical role in enabling the repair of healthy muscle following wear and tear, by helping to stabilise the membrane during contraction-induced damage. “When you lack the ability to crosslink muscle fibres, contraction-induced injury starts a repetitive cycle that ultimately leads to fat and scar and fibrosis in the muscle, and loss of function,” she said.
PHYSICAL EXERCISE AND MUSCLE DAMAGE IN BECKER MUSCULAR DYSTROPHY
Animal studies have shown the potentially deleterious effect of exercise in X-chromosome-linked muscular dystrophy (mdx) mice. However, John Vissing, Director of the U niversity of Copenhagen’s Neuromuscular Center, Denmark, said it was important to remember the limitations of these investigations, i.e., that they evaluated only eccentric exercise, in which the muscle lengthens, or electrical stimulation of the musculature, and that the effect has not been demonstrated in a clinical setting.6,7 While there are data to suggest that people with dystrophies are more susceptible to muscle damage than the general population,8 that does not mean they cannot derive similar benefits from exercise, if carefully planned.
In 2008, Vissing’s team conducted the first in-human study of endurance exercise in Becker.9 Eleven patients moderately affected by Becker and seven matched healthy controls cycled for 30 minutes, five times a week for 3 months. Six patients continued, training two or three times a week, for 1 year. Over the 12 weeks, fitness, as measured by maximal oxygen uptake and achieved wattage, increased in all patients. This improvement was sustained in those who continued for the 12 months. “Physiologically, this looks very reassuring. They get the same response as the healthy person,” said Vissing. The study also showed that cycling resulted in a mean 40% increase in knee extension strength among the patient group. “This is something you won’t see in a healthy person, and probably relates to the fact that they are really deconditioned when they start this exercise.” The regime also appeared to be safe, with levels of the muscle damage biomarker creatine kinase (CK) remaining stable across all phases of the study. Muscle biopsies performed in five patients found no signs of increased degeneration or satellite cell depletion, in line with the CK measurement findings. The paper concluded that it is feasible to train persons with BECKER. “It appears to be safe, the patients develop more endurance, they get stronger, and it seems to be long lasting, at least for a year,” said Vissing.9
Exercise in Weaker Patients with Duchenne Muscular Dystrophy
Vissing next debunked the general thinking that people with muscle strength of <10% of normal are “untrainable.” He pointed to a 2012 study of resistance training in people with limb‐girdle muscular dystrophies (LGMD) and Becker.10 It showed that even those with <20% of normal knee extension strength achieved significant improvements following 6 months of strength training.10 “This is something we have seen in a number of cases,” he added.
There are ways clinicians can help this patient group to take part in exercise training, such as anti-gravity treadmills, which use a lifting effect to support up to 80% of the person’s body weight and alleviate the impact of training on the muscles.11 In one study, eight patients performed 10 weeks of aerobic and strength training on an anti-gravity treadmill in Vissing’s laboratory. Six-minute walking distance, dynamic postural balance, and plasma CK levels were assessed 10 weeks prior to training, immediately before, and 10 weeks after the training. The study recorded an 8% increase in walking distance and a 13% increase in dynamic postural balance, suggesting improvements in physical function.11 Analysis showed strength training (squats, calf raises, lunges) demonstrated increases in closed-kinetic-chain leg muscle strength over 10 weeks.11
Assisted cycling may be useful for people who are wheelchair bound, Vissing added. He highlighted as yet unpublished data from a study of 19 people with muscular dystrophies at his centre. It suggests this model can improve cardiovascular fitness, and increase strength, though probably with minor functional importance, while alleviating the back and buttock pain associated with sitting, and the gastrointestinal symptoms related to immobilisation.
Dystrophies, Exercise, and Biomarkers
Vissing also spoke about biomarkers of muscle-injury response, and their potential role in the evaluation of disease progression. In a study by his team and Edgewise, nine people with Becker, eight with LGMD 2I, nine with LGMD2L, and nine healthy controls underwent a high intensity, bimodal aerobic and strength exercise regimen. Blood was taken before, during, and after exercise, and analysed for around 7,000 proteins. In the Becker and LGMD groups, there were 32 common elevated proteins, and one commonly decreased protein, at baseline. Of the 32 that were elevated, which included CK, all were expressed in the muscle tissue, and 25 increased further during exercise. The results, Vissing said, were validated using Becker samples from the Newcastle Tissue Bank Dataset. “We have found a common signature for these muscular dystrophies,” said Vissing.12
Upon further analysis, the researchers found proteins that responded to exercise appeared to decrease with age, while the non-responsive proteins followed the opposite trend.12 Vissing said: “Maybe the non-responsive biomarkers can be used for disease progression long term, whereas the responsive proteins can be used acutely, if you give a treatment here and now.”
PHYSICAL EXERCISE IN DUCHENNE MUSCULAR DYSTROPHY
Focusing on the appropriate type of exercise to prescribe to boys with Duchenne could help to balance the negative impact of muscle damage with the positive impact of physical activity, said Tanja Taivassalo, Associate Professor in the Department of Physiology and Aging at the University of Florida, Gainesville, USA.
Muscle adaption to exercise, she explained, relates to two factors: the intensity and the frequency of muscle contractions. “If a muscle is subjected to higher intensity, lower frequency contractions, it will elicit a signalling cascade that leads to hypertrophy and a stronger muscle. Conversely, if a muscle has more frequent, less intense muscle contractions, it will become much more fatigue resistant,” she explained. Aerobic exercise induces a signalling cascade that leads to a slow oxidative phenotype that results in more mitochondria, better antioxidant capacity, better calcium handling, more capillaries, and an increased utrophin expression along the sarcolemma. Preclinical studies have shown this signalling cascade is intact in the mdx mouse model.13 “Promoting this slow oxidative phenotype through strategies like aerobic training is a promising and physiologically relevant strategy. However, it hasn’t been widely accepted or applied to Duchenne.” This, she believes, is because most preclinical research has focused on downhill treadmill running, which elicits eccentric muscle contractions, or lengthening, known to be damaging to mdx muscle.14 Indeed, a study comparing muscle damage in mdx mice running on a horizontal treadmill to those running on a downhill treadmill found the former did not generate the same increases in MRI T2 as the latter.14 This, Taivassalo said, suggests that contraction-induced injury is dependent on the type of muscle contraction. “A consequence of these preclinical studies is that we understand that boys with Duchenne should not do eccentrically-focused exercise,” said Taivassalo.
Exercise Type
Isometric exercise, in which force is generated without a change in length of the muscle, does not expose the muscle to damaging eccentric contractions.15 In one study, eight boys with Duchenne completed a 12-week, remotely-supervised, mild-to-moderate intensity strengthening programme. There was no change in serum CK levels 48-hours after one ‘bout’ of the activity, or after multiple sessions. In addition, there was no increase in MRI T2 signal or pixel intensity, either 48 hours after the session, or after 12 weeks. At the end of the programme, there were improvements in peak knee extension and knee flexor strength. This translated to an improvement in function, with a decrease in the time taken to ascend/descend four stairs.15 “These results are really exciting because they show, for the first time, that dystrophic muscle in boys with Duchenne is capable of adapting to an appropriate overload,” said Taivassalo.
The 2013 ‘No Use is Disuse’ study was the first randomised controlled trial of assisted bicycle training in Duchenne.16 Ambulatory and recently wheelchair-dependent boys with Duchenne were allocated to an intervention or control group. Those in the former took part in 15 minutes of upper and lower limb assisted bicycle training five times a week for 6 months, while the control group did no exercise. After 24 weeks, the control group received the same intervention. The researchers recorded a 6% decline in motor function among the control group, which did no exercise, while function measures were stable in the intervention group.16 “They concluded that this type of exercise is safe and has the potential to delay functional deterioration, but they did not include any measures of muscle damage. Importantly, there was no information about how much work the muscles actually did, versus what the motor did in the ergometer,” said Taivassalo.
Meaningful Change
To address these questions, her team collaborated with the University of Florida’s engineering department, developing “an active cycling paradigm.” Feedback sensors in the peddles of the cycle ergometer allowed them to track the cadence and adjust the level of motor-assistance in real-time, depending on the patient’s abilities. Taivassalo said: “The boys were looking at a computer screen with a target intensity, dictated by their heart rate. If they were not able to reach the target, the motor kicked in to add some assistance, but if they were above the target, it applied some resistance. We were able to then capture how much work was done passively, by the motor, and how much work the boys did themselves.”
Six boys with Duchenne took the device home for a 6-month, remotely-monitored endurance training pilot study. The length of each session increased over time, and the intensity was set at 50–60% of their peak heart rate. Presenting the results ahead of publication, Taivassalo said there was no evidence of muscle damage after 6 months, as measured by CK at rest, or T2 signal. Fitness increased, with the level of assistance provided to each boy by the machine decreasing over the period of the study. Comparing baseline to 6-month data, each participant had an average 15–20 beats per minute lower heart rate at the same submaximal workload. Cardiopulmonary exercise testing showed improvements in time to fatigue, peak work, and peak aerobic capacity. Bone density, as measured by DEXA, was maintained, and the team is now planning further studies to explore impact on the rate of muscle fat accumulation.
Importantly, “pretty much every single parent” said quality of life had “somewhat” or “very much” improved as a result of the training. “The boys were much more independent, they engaged in activities more, more active in gym class, and they were just much more confident in things they were doing,” said Taivassalo, highlighting the story of one boy who “didn’t want to stop exercising.” The team installed the feedback system on his own tricycle, and he has since gone from only being able to cycle for 2 minutes to taking part in a 5 km run, on his tricycle, with his family. “He says this is a whole new world, because this is so meaningful to him.”
TARGETING PROTECTION AGAINST CONTRACTION-INDUCED INJURY IN BECKER: AN OVERVIEW OF THE SEVASEMTEN (EDG-5506) CLINICAL PROGRAMME
Controlled exercise can be beneficial in preserving and improving muscle function in Becker and Duchenne, and could be used as an adjunct to emerging treatments. Pharmacological modulation of fast muscle myosin, for example, “could protect against contraction-induced muscle injury,” said Donovan.
While no such agents are currently available, sevasemten, an investigational drug that is not approved in any territory, is currently in clinical development. Designed to protect the muscle fibres against contraction-induced damage, it is an orally administered, allosteric, selective, fast myofiber (Type II) myosin inhibitor.17 Donovan presented 24-month data of the ARCH study, ahead of publication.
History and Measures
ARCH is an open-label, single-centre study assessing the safety, tolerability, and pharmacokinetics of sevasemten, as well as its impact on muscle biomarkers. It used the North Star Ambulatory Assessment (NSAA), a well-established and validated 17-item rating scale used to measure functional motor abilities,18,19 as an outcome measure (Figure 1). Published data that was augmented by additional unpublished data from the Padova Becker Natural History Study20, presented by Luca Bello at the 2022 Muscular Dystrophy Association (MDA) conference, demonstrated that NSAA decline is consistent in people with BECKER who are already progressing, with individuals with a baseline score of between 10–32 experiencing an estimated decline of -1.22 NSAA points each year (unpublished data, Bello. L). These results were further validated by two natural history studies, which recorded a -2.5 point decline over 18 months and over 2 years.21,22
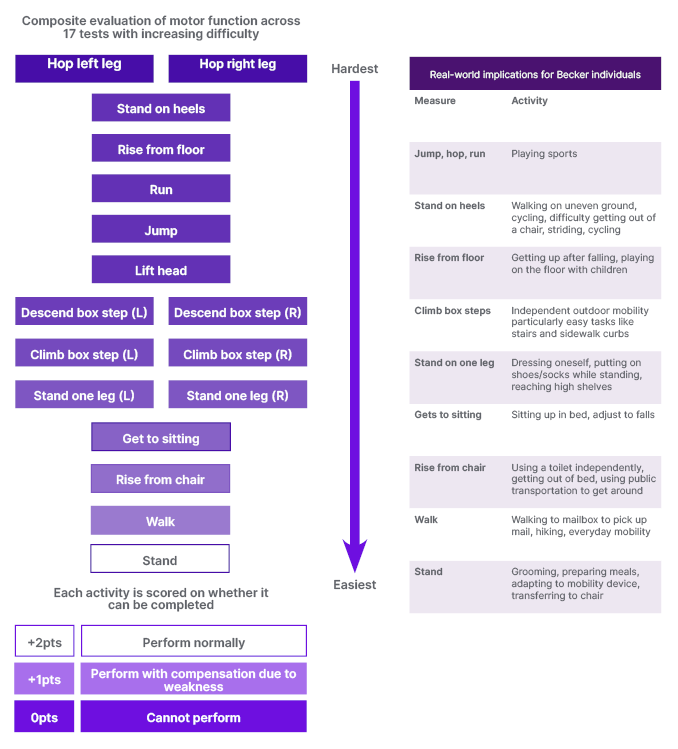
Figure 1: North Star Ambulatory Assessment (NSAA) and its real world implications.18,19
Putting this into context, Donovan said: “At high NSAA scores, there are some things people are compensating for, like getting off the floor or going on tiptoes, but they can do all the NSAA tasks. In a few years, when they reach a NSAA score in the 20s, they are starting to compensate. The natural history would predict that in another 8 years or so their NSAA would be in the teens. Those with a NSAA in the teens have started to lose meaningful functions such as being able to climb a step or rise from the floor.” In another 8 years, she said, natural history would predict that many NSAA functions will be completely lost. “What we need to do is find something that will stabilise them,” she added.
24-Month Data
ARCH was an open-label, single-centre study assessing the safety and pharmacokinetics of sevasemten in adults with BECKER. The study also assessed the agent’s longer-term functional affect. At baseline, the 12 ambulatory males, all aged 18–55 years, had an average NSAA of 15, increased serum CK, and decreased lean muscle mass. They were already functionally impaired, and “you would expect them to decline,” Donovan said.
The 24-month data showed that sevasemten was well tolerated at all doses, with no dose reductions or adjustments, no treatment discontinuations due to adverse events, and no serious adverse events. Over the 2 years, 11 of the 12 patients remained stable with regard to their NSAA score. “Whether they were at the top of the NSAA at 31, or were barely ambulatory, starting out with a score of four, they maintained function. In some cases, they even gained some function,” said Donovan. This contrasts significantly, she went on, with the approximately 2.4 point decrease that would be expected from the natural history studies (Figure 2). The one patient who experienced a decline in NSAA had suffered a meniscal tear that required surgery, and immobilised them post-operatively. Donovan said that this spoke to the role of continued exercise in Becker management.
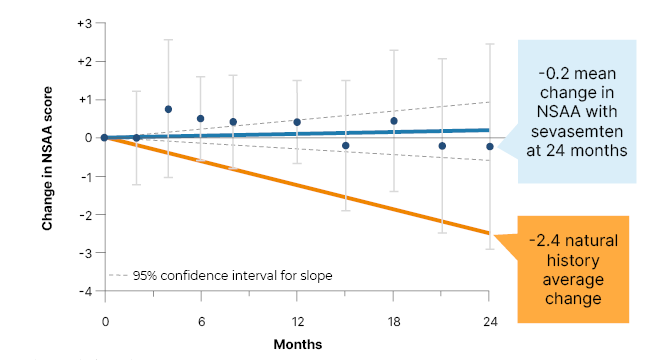
Figure 2: ARCH study data presented ahead of publication: North Star Ambulatory Assessment (NSAA) stabilised, diverging from natural history at 24 months.
*All data through 24 months, including patients recovering from meniscus surgery.
Natural history based on unpublished data presented by Bello at MDA and van de Velde et al.22
Mean ±95% confidence intervals.
NSAA: North Star Ambulatory Assessment.
Biomarkers of muscle damage that are elevated in people with Becker, including CK, fast skeletal muscle troponin I, and myoglobin, decreased rapidly after treatment initiation, and this decrease was maintained over the 24-month period. Other measures of muscle function, such as the 100 m timed walk test and maximum grip strength, remained stable, suggesting that inhibiting the fast myofiber (Type II) myosin inhibitor does not negatively impact muscle strength.
Maximal biomarker response was recorded at a 10 mg dose, and this finding has now been taken forward into a pivotal clinical trial (NCT05291091/ Grand Canyon). Edgewise is aiming to recruit 120 adult males diagnosed with Becker across the USA and Europe in their GRAND CANYON study. Key inclusion criteria are: aged between 18–50, a mutation in the dystrophin gene with Becker phenotype, and ambulatory with NSAA of between 5–32. The company is also looking at the agent’s safety and efficacy in Duchenne, Donovan added.
CONCLUSION
While contraction-induced injury underlies disease progression in Becker and Duchenne, this does not mean that exercise is not beneficial, and clear benefits have been observed. However, avoiding the type of exercise that is most associated with damage, i.e., eccentric contraction, is important in guiding exercise in muscular dystrophy. Additionally, approaches to pharmacologically protect against contraction-induced injury hold promise.