Abstract
Surgical site infections (SSI) are infections occurring within 30 days of the post-operative procedure. They are common post-operative morbid complications that may cause death if not treated timely. The common causes of SSI include infectious bacteria, such as Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and some Enterobacteriaceae.
This was a cross-sectional study conducted at St. Francis Referral Hospital, Ifakatra, Tanzania over a period of 12 months to investigate the causes of SSI and antimicrobial susceptibility of the causal agents. The study included consenting patients who developed post-operative wound infections during the study period. Identification of infecting micro-organisms and their antimicrobial susceptibility was done at St Francis Referral Hospital Laboratory. Antibiotic susceptibility tests of the isolates were performed by the Kirby–Bauer (K–B 1966) disc diffusion test, and extended spectrum β-lactamase producing Gram-negative species were tested by using the modified double disc synergy test.
A total of 130 patients developed post-operative wound infection. Third and fourth decades were the most affected age groups; females were the dominant group with a 1:1.4 male: female ratio. Out of the 130 specimens, 121 isolates were obtained, and nine specimens were negative for culture. P. aeruginosa was the most commonly isolated agent (42.1%), followed by S. aureus (19.8%), while the least were Streptococcus spp. at 0.8%. The isolates showed the highest resistance to ampicillin (91.7%), and least to ciprofloxacin (1.7%). P. aeruginosa was highly resistant to both amoxicillin + clavulanic acid (98%), and to ampicillin (98.0%). Extended spectrum β-lactamase E. coli producers were 68.4%.
The bacteria causing SSI require continuous monitoring to obtain data that will support local and national guidelines in the battle against antimicrobial resistance, and improve therapeutic outcomes following surgical interventions.
Key Points
1. Surgical site infection is a surgical challenge resulting in unfavourable surgical outcomes. The condition is more pronounced in low- and middle-income countries, where surveillance of diseases (including surgical site infection) is low. The major aim of this article is to meet the Global Sustainable Development Goal 3: ensuring health and well-being for all. In order to comply to the World Health Organization (WHO) antimicrobial resistance reduction target, the use of surveillance systems should be advocated for at an institutional level.
2. The manuscript explains the cause of wound infection in post-operative patients, and the profile of bacterial susceptibility. The study results showed that Gram-negative bacteria more frequently cause surgical site infection, and are also highly resistant to commonly used antimicrobial agents at the authors’ health institution.
3. The surgeon and clinicians should be aware of the presence of highly antimicrobial-resistant bacteria. The presence of extended spectrum β-lactamase-producing Escherichia coli indicates the need for evidence-based infection prevention and control at a local and national level.
INTRODUCTION
Surgical site infections (SSI) are defined as infections occurring within 30 days after simple skin incisions, or complex interventions involving subcutaneous tissue, organs, or manipulated space during primary operation.1-3 SSIs are common post-operative complications which may cause significant morbidity.4–7 Globally, the incidence of patients developing SSI post-surgery ranges from 15.45–23%, as reported by the World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC).8 Approximately 77% of post-operative deaths are reported to be related to post-operative infections.9 The condition is probably high in developing countries, but it is still difficult to quantify the burden due to the limited availability of data and publications.10,11
Causes of SSI differ from one region of the body to another. The most commonly reported bacteria in SSI are hospital-acquired infections, involving Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli and other Enterobacteriaceae.1,8,12-15 SSI prevention and control are currently challenged by the emergence of antimicrobial resistance, which in developing countries is aggravated by underlying poor health systems.15-17 Irrational antibiotic use and unavailability of SSI data are among the areas which need to be critically addressed to improve surgical services.18 SSI can occur in any surgical procedure ranging from obstetrics and gynaecology, to general surgery and orthopedics.19-22 SSIs complicate therapeutic outcomes associated with prolonged length of hospitalisation, and increased hospital costs.6,8,19
Multidrug resistant bacteria commonly include methicillin resistant S. aureus and extended spectrum β-lactamase (ESBL)-producing Gram-negative bacteria.23-26 The ESBL-producing Enterobacteriaceae, particularly E. coli, Klebsiella spp., and P. aeruginosa, have shown high levels of resistance, ranging from 50–100% for commonly used antimicrobial drugs, such as ampicillin, chloramphenicol, and third generation cephalosporin; while the same bacteria have been reported to have resistance below 25% to piperacillin-tazobactam and imipenem.9,27,28 Specific therapeutic options for patients with SSIs depend on data from antimicrobial sensitivity tests done by clinical laboratories. One zonal hospital in Tanzania reported an SSI rate of about 10.9% involving mostly S. aureus and Enterobacteriaceae.2,29 Tanzania, like most Sub Saharan African countries, lacks reliable evidence-based data on SSIs, particularly in rural health facilities, including referral hospitals.3,8 The aim of this study was to evaluate the SSI causative agents and their antimicrobial susceptibility at a local health institute.
METHODOLOGY
Study Site
A prospective hospital-based cross-sectional study was conducted from January 2022–December 2022 at St. Francis Referral Hospital, Ifakara, Tanzania, which is a rural faith-based private referral hospital. The hospital is also a teaching hospital for medical students from St. Francis University College and Allied Sciences (SFUCHAS). The hospital provides general and specialised surgical services, including orthopaedic, urologic, obstetric, and gynaecologic, and also offers haemodialysis.
Sampling Techniques
Non-probability sampling method was used for all patients who were operated on during the study period at St. Francis Referral Hospital. The patient particulars, diagnosis, and procedures were collected from the individual patient’s file after they developed a wound infection. There was a clinician who was responsible for data collection from the respective department, particularly general surgery, orthopaedics, obstetrics and gynaecology, outpatient clinics, and urology. Therefore, post-operative patients were subjected to a routine daily wound review. Early infections were identified during ward rounds, while late infections were identified after discharge. At the time of discharge from hospital admission, the patients were requested to attend outpatient clinic follow-up, weekly, for 4 consecutive weeks. The patients were also requested to provide their contact details for easy follow-up after discharge. In case of signs of wound infection while at home, the patients were requested to return to the hospital for clinical evaluation. Each patient was discharged from post-surgical follow-up 30 days later, when free of wound infection and clinically stable. The patient who developed post-operative wound infection was informed about the study, and was requested to participate in the study. Two patients were excluded from the study: one refused to sign the consent form, and the second was lost from the follow-up after discharge. Identification of SSI was according to the clinical criteria for SSIs, and was classified according to the CDC for SSI Classification System (superficial incision SSI, deep incision SSI, and organ/space SSI).24,30,31 Specimens were collected by swabbing the infected wound before antiseptic dressing to avoid skin contamination. The swabs were then immersed in a container with peptone water pH transport medium,32,33 and sent to the bacteriology laboratory.
Processing of Specimens Culture and Bacterial Identification
The specimens were inoculated by streaking on McConkey, and mannitol salt agar with egg yolk media, then sub-cultured on blood agar and incubated at 35–37°C for 24 hours. Isolates were identified by colonial morphology, Gram staining, and conventional biochemical tests, including catalase, coagulase, mannitol fermentation, and haemolysis for Gram-positive bacteria; and for Gram-negative bacteria, colony morphological features and biochemical tests, including urease, citrate, oxidase, indole, and sugar utilisation on triple sugar iron.
Antimicrobial Susceptibility Testing
Antibiotic susceptibility tests of the isolates were performed by the Kirby–Bauer (K–B 1966) disc diffusion test.32 From a pure culture, three to five colonies of bacteria were picked using a sterile loop, and soaked into a tube with 5 mL of normal physiological saline, to obtain a density equal to 0.5 MacFarland scale, and then seeded culture evenly over the entire surface of sterile Mueller–Hinton agar plate (Oxoid, UK). The plates were then kept at room temperature to dry, and then antibiotic discs (Oxoid, UK) were placed and incubated at 35–37°C for 18–24 hours. The discs tested were from the commonly used antibiotics at the hospital, including ampicillin (10 μg), clindamycin (15 μg), vancomycin (30 μg), amoxicillin-clavulanic acid (20/10 μg), gentamicin (10 μg), ceftriaxone (30 μg), ciprofloxacin (5 μg), meropenem (10 μg), and piperacillin-tazobactam (100/10 µg).34
ESBL-producing Gram-negative species were tested by using the Modified Double Disc Synergy Test (MDDST) using a disc of amoxicillin-clavulanate (20/10 μg) along with meropenem (10 μg), ciprofloxacin (5 μg), ceftriaxone (30 μg), and piperacillin-tazobactam (100/10 μg). The use of fourth generation of cephalosporins was not included in this study as it is recommended for MDDST, because these group of drugs are not commonly used in the authors clinic set-up.35 Any increase in the zone towards the disc of amoxicillin-clavulanate was considered positive for ESBL production.12,25,36
The quality control of the laboratory works was maintained through aseptic and sterility conditions, labelling, and storage in recommended environmental conditions.19,32 International control bacteria strains, E. coli ATCC25922, P. aeruginosa ATCC 27853, and S. aureus ATCC 25923 were used.12,32,37
Inclusion Criteria
All patients who signed the consent form to participate in the study voluntarily were included.
All patients who were operated on during the study period, and developed a wound infection during the study period, were included.
Exclusion Criteria
The exclusion in this study was based on involuntary consent of the patient, with congenital or acquired speaking impairment such as aphasia autism (due to a language barrier and lack of special group language interpreters in the authors’ area).
All patients who withdrew from the study were also excluded.
Other exclusion criteria were ophthalmological, ear, nose, and throat surgical procedures, as these particular procedures were not commonly performed at the authors’ set-up during the study period due to a lack of specialised clinicians in these fields.
Data Management and Analysis
Data was collected from the patient after follow-up during post-surgical care for wound progression, and laboratory results by using data-collecting tools. Data were entered into a statistical package for the Social Sciences 26 version. After this data entry, cleaning was computed by running frequency. Nominal variables including isolate names were compared with antibiotics to determine sensitivity and resistance patterns. Multivariate logistic regression was used to analyse the factors associated with post-operative wound infection. The odds ratio test was used to verify the correlations between variables and the SSI rate. In the logistic regression analyses, the variables included age, sex, department, procedure, and wound. Estimation of odds ratio and 95% confidence interval (CI) was performed for selected variables. A P-value <0.05 was considered statistically significant.
STUDY LIMITATIONS
The study was performed within a rural hospital where advanced clinical laboratory diagnostic facilities are limited. There was a time limitation of 12 months depending on the project period.
RESULTS
A total of 1,763 patients were operated on during the study period, and out of this cohort, 130 patients developed post-operative wound infection (7.4%). Among them, 41.5% (54/130) were male, and 58.5% (76/130) were female, with a 1:1.4 male-to-female ratio. The most affected age groups were within the third and fourth decades, making up 57% of all who developed SSIs. The overall mean age was 34.0±15.7 years. Most of the patients were from general surgery (43.08%; 56/130), whereas 37.0% (44/130) were from the obstetric ward. Laparotomy was the most common procedure at 44.8% (58/130); other procedures were caesarean section at 37.0% (48/130); open reduction and fixation at 8.5% (11/130); and other various procedures made up 11.54% of these patients (15/130), including herniorrhaphy (4), thyroidectomy (1), Mayo’s repair of umbilical hernia (3), hydrocelectomy (2), donor site for skin grafting (2), subcutaneous cystic excision (1), open prostatectomy (1), and plate removal (1). Most of the wounds were clean (50.0%; 65/130) 26.15% of wounds (34/130) were dirty, and 19.23% (25/130) were contaminated.
Out of 130 cultured specimens, 121 were positive and nine were negative. Most of the patients who developed SSI were post-operative patients from general surgery, accounting for 44.63%, followed by obstetric-caesarean surgical cases accounting for 38.84% (Table 1).
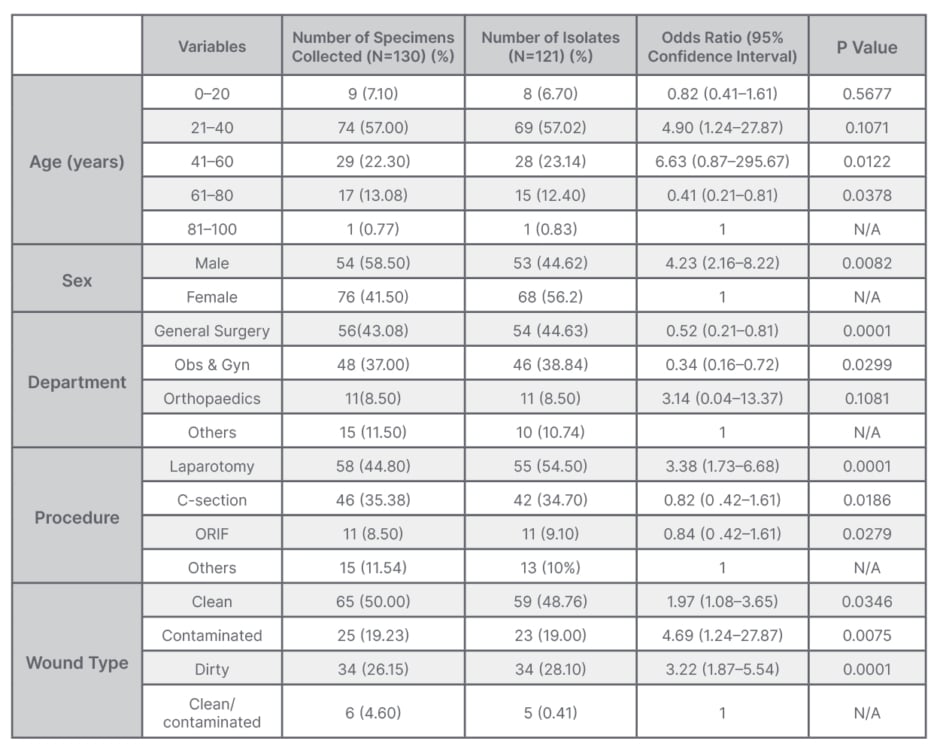
Table 1: Association of clinical and demographic characteristics of patients who developed wound infection.
N/A: not applicable; Obs & Gyn: obstetrics and gynaecology; ORIF: open reduction and internal fixation.
Gram-negative bacteria, particularly P. aeruginosa, was the predominant isolate at 42.1% (51/121) followed by S. aureus at 19.8% (24/121), E. coli at 15.7% (19/121), and K. pneumonia at 13.2% (16/121). Other isolates were Proteus spp at 5.0% (6/121), Bacillus spp at 3.3% (4/121), and Streptococcus spp at 0.8% (1/121) (Table 2).
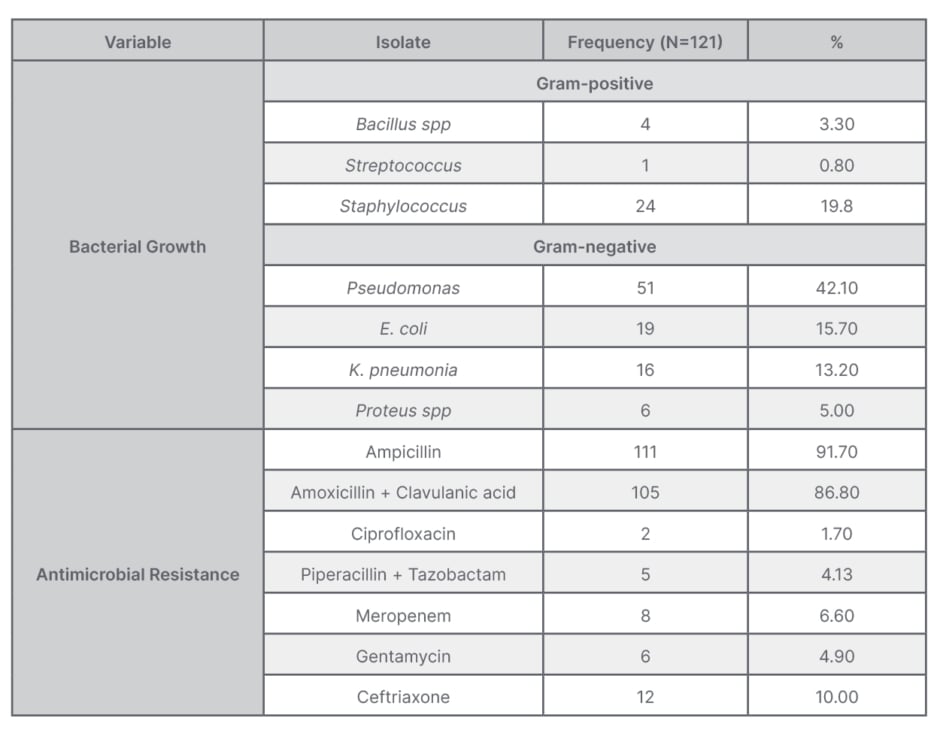
Table 2: Distribution table of bacterial growth and antimicrobial resistance.
The overall resistance to ampicillin was 91.7%, followed by amoxicillin + clavulanic acid at 86.8%. The least resistance was against ciprofloxacin at 1.7%, while antimicrobial resistance rate against piperacillin + tazobactam was 4.13%, gentamycin 4.9%, meropenem 6.6%, and ceftriaxone 10.0%. On multivariate logistic regression analyses, history of laparotomy and dirty wound were significantly associated with development of wound infection (Table 3).
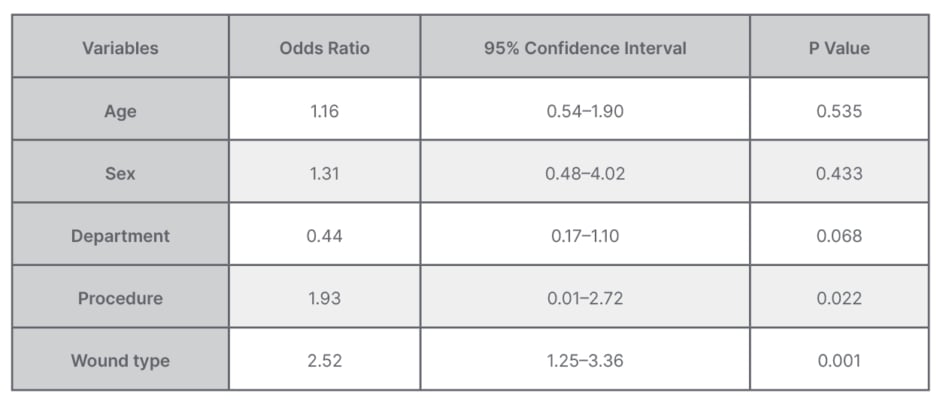
Table 3: Multivariate logistic regression analysis for factors associated with bacterial isolation from surgical site infections.
Antimicrobial Susceptibility Testing
Staphylococcus was significantly resistant to amoxicillin + clavulanic acid (79.2%), and to ampicillin (87.5%), but sensitive to ciprofloxacin (91.6%), piperacillin + tazobactam (95.8%), meropenem (100%), and gentamycin (87.5%). Pseudomonas was highly resistant to amoxicillin + clavulanic acid (98.0%) and ampicillin (98.0%), but less resistant to piperacillin + tazobactam (5.8%), meropenem (7.8%), and gentamycin (1.2%). Other antimicrobial susceptibility patterns are summarised in Table 4.
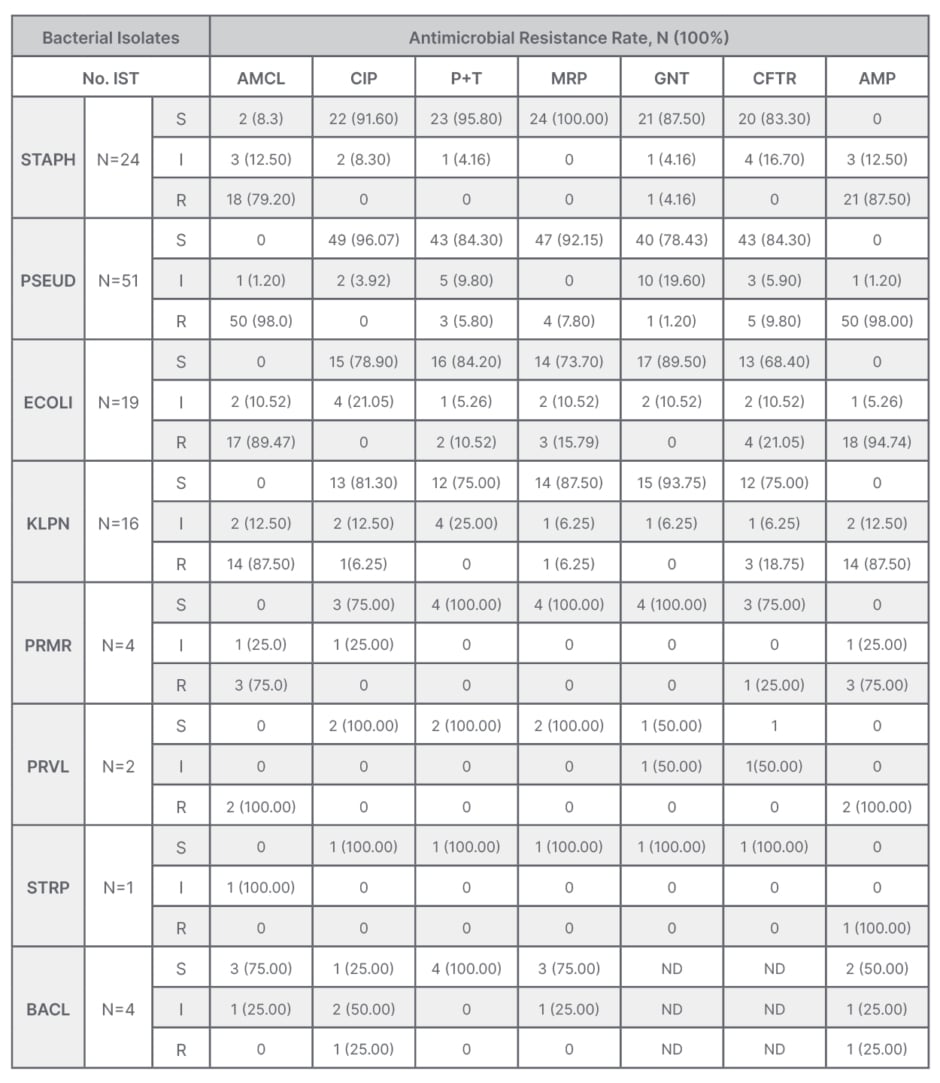
Table 4: Pattern of bacterial isolates and antibiotic resistance profile among isolates from post- operative wounds.
AMCL: amoxicillin + clavulanic acid; AMP: ampicillin; BACL: Bacillus spp; CFTR: ceftriaxone; CIP: ciprofloxacin; ECOLI: E. coli; GNT: gentamycin; I: intermediate; KLPN: K. pneumoniae; MRP: meropenem; ND: not done; No. IST: number of isolates; P+T: piperacillin+ tazobactam; PRMR: P. mirabilis; PRVL: P. vulgaris; PSEUD: Pseudomonas; R: resistant; S: sensitive; STAPH: Staphylococcus; STRP: Streptococcus spp.
ESBL Testing
Of the 19 E. coli isolates, 68.4% (13/19) were found to be ESBL producers by MDDST. Among these, seven isolates showed a clear edge of inhibition of ceftriaxone towards amoxicillin-clavulanic acid. Otherwise, all 13 E. coli strains were sensitive to ciprofloxacin and meropenem.
DISCUSSION
In this study, the reported prevalence of SSIs (7.4%) is higher than previously reported in a number of developing countries, which ranged from 2.3–4.5%.1,3,6,26,38,39 The results of this study agree with those of other researchers.18,37,40 This study has further shown that Gram-negative bacteria are the major causes of SSI, with P. aerugenisa being the predominant pathogen, accounting for 42.1% of all SSIs, consistent with other studies.7,40,41 Other causes are S. aureus (19.8%), E. coli (15.7%), K. pneumoniae (13.2%), Proteus spp (6.6%), and Bacillus spp (3.3%). The Bacillus spp described in this study should not be considered as a contaminant, since they were isolated from the infected wound, as reported in other studies as well.13,28,42-44
The sources of the pathogens shown by this study include the patient’s own indigenous flora, the hospital environment, health workers, and visitors, as reported elsewhere.4 However, the findings of this study differ from others which reported E. coli and S. aureus as the major causes of SSI.2,6,14,15,29,41,45,46
In the authors’ study, all patients received pre-operative antibiotic prophylaxis, consisting of ampicillin and cloxacillin, or ceftriaxone with metronidazole; however, 7.4% of patients developed a SSI, which is lower than other studies, which report a SSI prevalence of 14.8–59.0%.8,9
Despite the national program on quality improvement in Tanzania’s health facilities, post-operative wound infections are a significant challenge for surgeons.9,19,47 Introducing infection prevention and a control pre-operative check list, and pre-operative antibiotic prophylaxis in surgical practice would be helpful to reduce further post-operative adverse outcomes.16,17,24,29-31,48,49
SSIs still occur at high rates in low- and high-income countries alike. For instance, 0.7 infections occur per 100 open procedures in the USA.21,38,50 Therefore, there is a need to review prophylaxis regimes locally, including the timing. According to the CDC guidelines, administration of antibiotic prophylaxis should be within 60–120 minutes before the skin incision is made.5,17,49,51,52 However, repeated dosage is recommended in case of prolonged procedures, and depending on the antimicrobial agent’s half-life.4,17,53 An overwhelming number of resistance cases to ampicillin and amoxicillin + clavulanic acid was observed in this study. This may be attributed to the irrational use of these drugs by the community, since these drugs are commonly dispensed by drug sellers and dealers over the counter. This is a regulatory challenge which needs to be seriously addressed in Tanzania.
The study also showed that there is a moderate bacterial resistance to piperacillin + tazobactam (4.13%), gentamicin (5.00%), meropenem (6.60%), and ceftriaxone (10.00%). Similar observations have been reported by other authors.15,16,25,26,40 This may be due to the reduced prescription of these drugs, because of their relatively higher price, and restriction of their availability in the essential drug shops (as known in Swahili words “Maduka ya dawa muhimu”). Regardless of the low rate of bacterial resistance to piperacillin + tazobactam, meropenem, and ceftriaxone shown in this study, this remains a ‘red flag’, with a possibility of therapeutic failure of these drugs in the future.
It is important that clinicians perform a thorough identification of pathogens, and choose the right antimicrobial agent for the management of bacterial infections. Antibiotics are fundamental in clinical practice, whether prophylactic or therapeutic. Drug availability, selection, and rational use constitute a triangle of global efforts against antimicrobial resistance.7,12,14,17 The correct antimicrobial selection must be based on correct bacterial identification and its susceptibility rather than the clinician’s choice.5,34,49,53
This study has shown a significantly high level of quinolones efficacy, particularly ciprofloxacin, which encountered an overall resistance of 1.7% by SSI pathogens. This has been shown by other studies to be the most powerful drug, with high antimicrobial potential.2,15,19,37 However, it should be noted that its level of resistance is increasing, and precautions are needed.26,29,34,41
From this study, the authors observed antimicrobial resistance to the so-called ‘stable drugs’, including meropenem (6.6%) and piperacillin + tazobactam (4.13%). This has also been demonstrated in other studies.1,19 Further studies are required on emerging carbapenemase-producing Gram-negative strains,28,54 particularly K. pneumoniae, P. aeruginosa, and some Enteobactericiae.40,49,55,56
The ESBL-producing E. coli (68.4%; 13/19), observed in this study correlate with reports from previous studies.13,15,24,25,45,57 However, ESBL-producing bacteria have been reported to exhibit resistance to non-β-lactam antimicrobial drugs.25,28,49,58 They counter the therapeutic effect, thus narrowing the range of treatment of choice.15,18,21 However, the high frequency of ESBL among E. coli requires additional research focusing on factors contributing to this emergence, which possibly could be the inappropriate use of antimicrobials, ill-equipped diagnostic laboratories, or non-adherence to guidelines on diagnosis and therapeutic approach for infectious diseases.
ETHICAL CONSIDERATIONS
The study was approved by the St. Francis University of Health and Allied Sciences Internal Reviewer Board (IRB), and ethical clearance was obtained from the National Institute of Medical Research (NIMR). Confidentiality was guaranteed by using identity numbers rather than the names of patients.
CONCLUSION
Conclusively, there is an increase in post-operative wound infections caused by antimicrobial-resistant pathogens. The most commonly identified organisms were P. aeruginosa, S. aureus, E. coli, and K. pneumoniae. Resistance to the most commonly used antibiotics, particularly third-generation cephalosporin, ampicillin, amoxicillin + clavulanic, and meropenem, is an alarming sign which should be responded to, in order to tackle this effect. In the authors’ study, ciprofloxacin and gentamycin showed effect in post-operative infection-causing agents. There is a significant need for adherence to the ‘reserve drug’ concept to reduce misuse of available antimicrobial drugs.
RECOMMENDATIONS
The SSI bacterial diversity demonstrated by this study should be a matter of concern, and it calls for strict adherence to infection prevention and control in hospital surgical wards. This study also calls for continuous interpretation of antimicrobial sensitivity tests at a local level to guide the choice of effective drug prescriptions. Furthermore, ‘over-the-counter’ dispensing should be strongly condemned and punishable by the regulatory bodies in the country. The National Treatment Guidelines for Antimicrobial Use in Infectious Diseases, and the Surgery Safety Checklist should be reviewed periodically for adherence and applicability at all levels of health facilities where surgery is commonly practised. This also includes pre-operative antimicrobial prophylaxis timing, and close post-operative care. Future studies are recommended at the molecular level for bacterial strains that are ESBL producers. Studies on environmental health safety, such as healthcare-related infections and hospital-acquired infections, are also recommended.







