Abstract
Background: Despite the advancements in the management of psoriasis (PsO) and psoriatic arthritis (PsA), there remains a significant delay to diagnosis of PsA. National guidelines recommend regular screening of patients with PsO for PsA. The aim of this study was to increase the screening of patients with PsO, and reduce the delay to diagnosis in PsA.
Methods: A retrospective baseline audit was conducted in patients with PsO attending a large general practice (Brookside Group Practice, Reading, UK) between November 2022–April 2023. In the follow-up stage between May–November 2023, a digital Egton Medical Information System (EMIS; Emis Health, Rawdon, Leeds, UK) web template, using the Psoriasis Epidemiology Screening Tool (PEST) questionnaire was implemented. The number of patients with PsO screened for PsA, and the number of newly diagnosed PsA cases, were recorded.
Results: At baseline, 15 patients with PsO were identified, and none had PsA screening done. In the follow-up phase, 28 patients coded for PsO were identified. There was an increase in the number of patients screened for PsO from 15 to 28, representing an increase of 87% from baseline.
From the follow-up group, 12 (43%) patients were screened for PsA. These patients were referred to the specialist clinic, and seven (58%) had confirmed PsA. This represented a population of patients with previously undiagnosed PsA within the general practitioner (GP) surgery.
Conclusions: The authors have successfully implemented an integrated and interactive digital screening tool for PsA within the GP system. This has led to an increase in the detection of patients with PsA. This practical and effective approach is in line with national guidelines for early detection, to prevent long-term damage and disability from PsA.
Key Points
1. Increasing screening for PsA in patients with psoriasis (PsO) at primary care level using digital interventions can improve identification and referral of potential psoriatic arthritis (PsA) at an early stage.
2. The screening tool optimised patient education opportunities for possible symptoms of PsA in patients presenting with PsO, so that they could return to the general practitioner if the relevant symptoms developed.
3. The concept of this paper is to show that small changes done within quality improvement cycles can bring positive clinical impact, that may be used in other areas as well.
INTRODUCTION
Psoriasis (PsO) is a chronic inflammatory skin condition that affects around 2% of the population in Europe.1 Psoriatic arthritis (PsA) can affect up to 30% of patients with PsO, and has a prevalence of around 0.37% in the UK.2 Despite the recent advancements in the management of PsA,3 there remains a delay to diagnosis, with a median time of 2.5 years.4 Reducing the delay to diagnosis of PsA is important, as joint damage can occur as early as 6 months from onset of symptoms.5 Half of people with PsA can develop irreversible joint damage within 2 years.6 Earlier treatment of patients with PsA using a treat-to-target approach aiming for remission or low-disease activity can result in better clinical outcomes.7
The majority of patients with PsA present with preceding PsO. When presenting with PsO in primary care, there is a lack of screening for underlying joint symptoms, contributing to the delay in diagnosis for PSA, where up to 85% of patients with PsO who are followed up in secondary care have undiagnosed PsA.8 To improve the earlier detection of PsA, there is a need to address the unmet need of enhancing clinicians’ awareness, through the use of a screening tool. The national guidance in the UK recommends annual screening for PsA in patients with PsO.9 The use of the Psoriasis Epidemiology Screening Tool (PEST) has been recommended by the National Institute of Clinical Excellence (NICE) in the UK. The PEST questionnaire is a validated screening tool, and a score of 3 or more out of 5 indicates a referral to rheumatology should be considered.10 Other screening questionnaires have been used in primary care, and there are minimal differences in the sensitivity and specificity for the detection of PsA.11 The use of screening questionnaires in real-world primary care clinics are dependent on simplicity and easy access, for both patients and clinicians.
Rationale and Aims
The objective of the study was to increase screening for PsA in patients with PsO at primary care level, with the implication to identify potential PsA at an early stage. A secondary objective was to utilise patient education opportunities for possible symptoms of PsA in patients presenting with PsO, so that they could return to the general practitioner (GP) if any of the relevant symptoms developed. The authors evaluated the use of the PEST screening tool, which was integrated into the GP system in the follow-up audit. The earlier referral to the rheumatology clinic for these patients would be in line with the PsO guidelines. The ultimate aim then would be for earlier referral for possible PsA in patients with PsO.
METHODS
The authors conducted this study within the collaboration set up between primary and secondary care, called the Rheumatology Academy and Collaborative Network (RheumACaN). This network provides training and mentoring of primary care clinicians through collaboration with secondary care rheumatology specialists.12 Using a quality improvement approach, which utilises the Plan, Do, Study, Act (PDSA) cycle, the authors evaluated the presence and effectiveness of PsA screening in patients with PsO (Table 1). This study was done within a service improvement framework, and had institutional approval. The authors planned (P) the study by carrying out a baseline audit to evaluate the existing practice of screening for PsA in PsO, prior to any improvement interventions. An audit was carried out at baseline for a 6-month period between the start of November 2022 to the end of April 2023. A retrospective case note analysis of patients with any form of PsO in a large GP surgery (Brookside Group Practice, Reading, UK), who attended the clinic during this period, were analysed. The case notes were reviewed to ascertain if screening for PsA had been undertaken during the clinic consultation for patients with PsO.
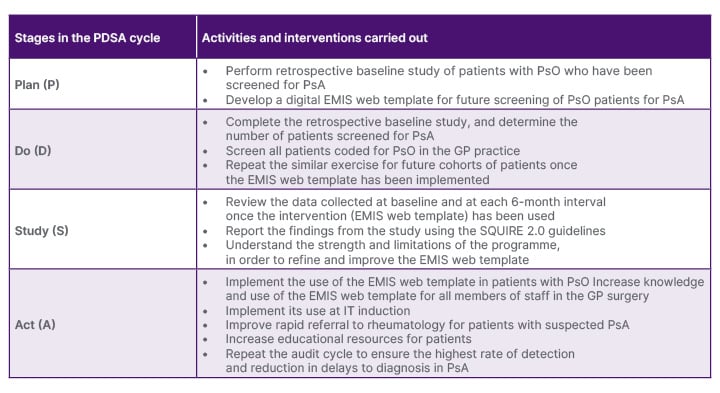
Table 1: The Plan, Do, Study, Act cycle used for the quality improvement programme to increase the screening and diagnosis of psoriatic arthritis in patients with psoriasis.
EMIS: Egton Medical Information System; GP: general practitioner; PDSA: Plan, Do, Study, Act; PsA: psoriatic arthritis; PsO: psoriasis.
Following the baseline audit, in the Do (D) stage, the authors implemented a new screening system in the GP system. Using the PEST questionnaire, which was integrated into the GP web-based system, Egton Medical Information System (EMIS; Emis Health, Rawdon, Leeds, UK), in a large urban GP surgery, patients with PsO were screened for the possible presence of PsA. A total score of 3 or more out of 5 from PEST was positive, and indicated a review of the patient for possible PsA. There was an assessment of patients who had possible PsA, including examination of skin and joints. In addition to the five questions from PEST, the authors also added two additional questions on the type of PsO, namely nail and scalp involvement, as these were likely predictors of PsA. The screening questionnaire was available for all members of staff at the GP surgery to use (Figure 1). Patients with PsA already known to secondary (specialist) care were excluded from the study.
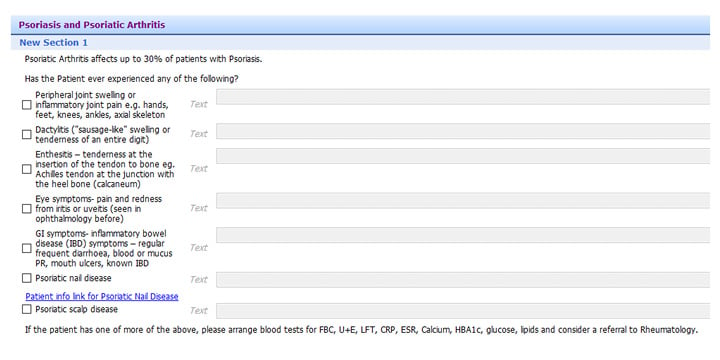
Figure 1: The integrated screening tool for psoriatic arthritis in patients with psoriasis.
This was integrated into the GP practice EMIS web template used for screening for PsA in patients with PsO.
EMIS: Egton Medical Information System; PsA: psoriatic arthritis; PsO: psoriasis.
In the Study (S) phase, the authors evaluated the number of patients with possible PsA detected, and the effectiveness of the digital screening system for PsA. In the Act (A) phase, they refined the digital system, and added in induction and educational programmes to enhance the detection of PsA in patients with PsO. The authors used the Standards for QUality Improvement Reporting Excellence 2.0 (SQUIRE 2.0) guidelines for reporting the key components of this systematic effort to improve the quality, value, and impact of their intervention.13
RESULTS
The GP surgery has around 30,000 patients registered, and had a good representation of the different age, sex, and ethnic groups represented. The GP database was screened for patients with PsO. At baseline, patients attending the surgery between November 2022–April 2023 were screened retrospectively for PsO. A cohort of 15 patients with PsO were identified. Of this group of patients with PsO, none (0%) were documented as having been screened for PsA (Figure 2a).
In the follow-up period, patients with PsO attending between May–November 2023 were screened for PsA following the implementation of the EMIS web template. In this follow-up audit, a cohort of 28 patients with PsO were identified. From the 28 patients with PsO, 12 (43%) patients were screened for PsA. In eight of the 12 patients (67%), the EMIS web tool was used to screen patients with PsO for PsA (Figure 2b). All 12 patients were referred to rheumatology clinic, and from this, seven (58.3%) patients had a confirmed diagnosis of PsA.
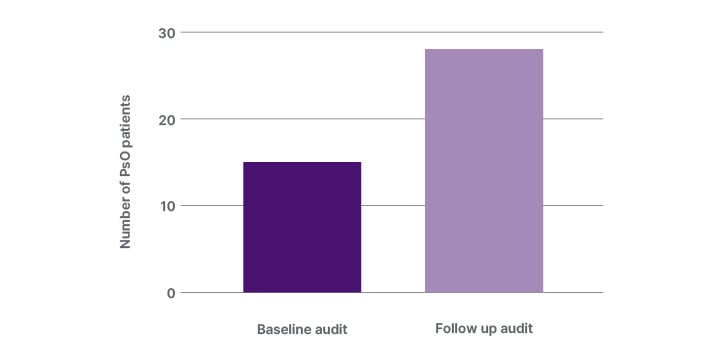
Figure 2a: The number of patients with psoriasis identified at baseline and at follow-up after implementation of the EMIS web template quality improvement programme.
EMIS: Egton Medical Information System; PsO: psoriasis.
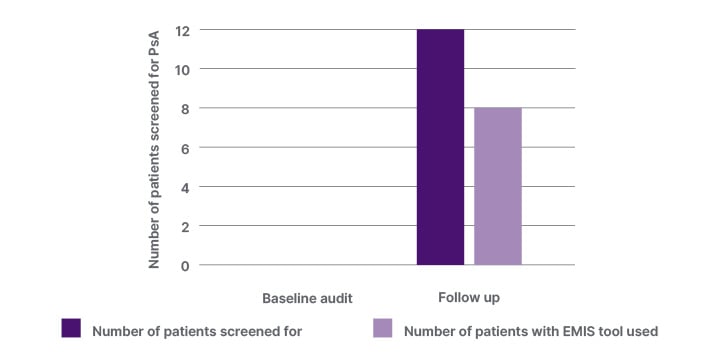
Figure 2b: The number of patients with psoriasis screened for PsA, and the number of patients with the EMIS web template used for screening for PsA.
EMIS: Egton Medical Information System; PsA: psoriatic arthritis.
Comparing the follow-up study using the screening tool with baseline, there was an increase in the number of patients screened for PsO from 15 to 28, representing an increase of 87% from baseline. The number of patients with PsO screened for PsA and referred had increased from zero to 12 patients at baseline and follow-up, respectively.
DISCUSSION
Patients with PsO often experience musculoskeletal (MSK) symptoms preceding the diagnosis of PsA. These MSK symptoms are often referred to as the psoriatic march leading to the development of PsA. There is increased prevalence rates of MSK symptoms and burden experienced by patients newly diagnosed with PsO through 5 years of follow-up.14 This presents an opportunity for screening of patients with PsO for PsA, leading to earlier diagnosis, and preventing long-term joint damage and functional limitations. More recently, the European League Against Rheumatism (EULAR) have produced points to consider for definition of clinical and imaging features suspicious for the progression from PsO to PsA.15 In children, juvenile spondyloarthropathies are a heterogeneous group of diseases, and, based on the International League of Associations Rheumatology (ILAR) classification criteria, patients with juvenile spondyloarthropathies are mainly classified under enthesitis-related arthritis or psoriatic arthritis groups.16 MSK symptoms, such as arthralgia, joint, and entheseal pain in people with PsO should be considered as a risk factor for PsA development. These features should be assessed regularly, and, if present, referral to a rheumatologist should be considered. Imaging such as ultrasound and MRI in people with PsO can also be used to help identify those at risk for PsA; in particular to detect synovio-entheseal involvement or abnormalities. These clinical and imaging features may help define patients with PsO suspicious of progression to PsA.
Different screening questionnaires for PsA in patients with PsO have been used in primary care. These include the PEST, the 8-item questionnaire CONTEST, and CONTEST with a minikin (CONTESTjt).17 Minimal differences in discriminative ability between these three screening questionnaires were found. The choice of which instrument to use will depend on other factors, such as simplicity and low patient burden.18 Other PsA screening questionnaires include Psoriatic Arthritis Screening and Evaluation (PASE),19 and the Toronto Psoriatic Arthritis Screen (ToPAS),20 which have been utilised in dermatology clinics.21 The Early Psoriatic Arthritis Screening Questionnaire (EARP) has also been used in clinics.22 There is also a need to develop screening questionnaires for diverse languages and cultures. One example is the Italian PsA screening tool, the Screening Tool for Rheumatologic Investigations in Psoriatic Patients (STRIPP) questionnaire.23 Developed from PASE, the STRIPP questionnaire had a higher sensitivity (0.92) and specificity (0.93) compared to the Italian version of PASE, with a sensitivity and specificity of 0.73 and 0.76, respectively.
The presence of numerous screening questionnaires for PsA reflects the heterogeneity and the various different domains of the condition, which also requires a systematic and joined-up multidisciplinary approach in its implementation in clinics. Clinical assessment is particularly important, as there may be other conditions that mimic PsA when the screening questionnaires are applied. The most frequent PsA-alternative diagnoses were osteoarthritis and fibromyalgia.24
A meta-analysis of psoriatic arthritis screening25 showed that the pooled sensitivity and specificity for the most commonly reported questionnaire-based screening tools (ToPAS, PEST, PASE, and EARP) ranged between 0.65–0.85, and 0.68–0.85, respectively. EARP was the most accurate screening tool, with the highest sensitivity and specificity (0.85 each), including when only high-quality studies were included. However, with further evidence and direct comparisons with other screening questionnaires, EARP’s accuracy was not comparably higher than that of the other questionnaire screening tools. The performance of the screening tools for PsA in primary care was evaluated in a cross-sectional study.26 Comparing the PEST, PASE, and EARP questionnaires for detecting PsA among patients with PsO in primary care, the PEST questionnaire has the most favourable trade-off between sensitivity (0.67) and specificity (0.71) to screen for PsA. EARP had a favourable sensitivity (0.87), but lower specificity (0.34). For this reason, the authors used the PEST in their study.
The implementation of the EMIS web template has been a beneficial intervention for increasing the number of patients with PsO who are screened for PsA. During the second 6-month period of the study, between May–November 2023, when the intervention took place, the number of patients with PsO who were screened had increased by 67% compared to baseline. The early identification of these patients with suspected PsA will allow for rapid referral to the early inflammatory arthritis clinic, which has a waiting time of less than 3 weeks. The case finding of seven previously undiagnosed patients with PsA is a significant achievement, as a result of the intervention put in place. In the authors’ model, this has improved the screening for PsA compared to baseline, where there was no documentation of this.
In a survey of clinical practice, 81% of dermatologists use PsA-specific screening instruments, while only 26.8% of rheumatologists use PsA screening tools to assess patients referred to them from all sources.27 In the authors’ study, 67% of patients with PsO who were screened for PsA had this done using the EMIS web template. This represents an improvement in detecting undiagnosed PsA compared to baseline, where none of the patients with PsO were screened for PsA.
The EMIS web template also served as a prompt for patient education. Increasing awareness and knowledge of PsA through patient education can help reduce delays to diagnosis, with patients with PsO reporting MSK symptoms to the clinicians earlier. The use of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA)’s treatment recommendations and educational resources can be helpful in this regard.28 The EMIS web template can signpost clinicians to these educational resources. It increases the awareness of the need for PsA screening, as well as for comorbidities, including cardiovascular screening in patients with PsO.
The template helped both clinicians and patients with PsO to recognise the possible MSK features that may signify the presence of PsA. This served as a prompt for the early referral of patients with PsO meeting criteria within the PEST questionnaire to the rheumatologists. The re-audit demonstrated an improvement in opportunistic screening for PsA in patients presenting with PsO. The screening tool is used as a pop-up or reminder when patients present with PsO in the clinic. There is potential to increase the use of PsA screening in patients presenting with PsO, and interventions employed include repeat presentations at clinicians’ meetings to increase awareness. There is also now the inclusion of the new template in new clinician IT inductions.
Screening of patients for PsA has been shown to be cost-effective compared to no screening. A study in Canada has shown that implementing screening in patients with PsO was expected to represent a cost saving of 220 million CAD per year, and improve the quality of life.29 Implementing a screening strategy such as the authors’ will be important, as the incidence of PsA is likely to rise in the future. A recent study in Germany predicted higher numbers of PsA in the coming decades if preventive strategies are not implemented. In the long term, it is important to implement preventive strategies to identify predictors, and treat PsO symptoms early, in order to delay, or even prevent, the transition of psoriasis to PsA.30
The limitations of the study are that the authors only screened patients with PsO attending the GP surgery during two 6-month periods, from November 2022–November 2023, hence the small sample size. They plan to screen all patients with PsO for features of PsA from the GP database in future. This will increase the number of patients screened and diagnosed with PsA. Not all patients with PsO were screened for PsA following the implementation of the EMIS web template (16 out of 28 were not screened), showing that further clinician training, induction, and awareness that is planned will be required. This is part of the quality improvement programmes, with repeat PDSA cycles, to continually improve the detection of PsA in patients with PsO.
The strengths of the study are that this EMIS web template has been implemented in real-world GP surgeries, with improvement in the screening of PsA from baseline, and improved training for clinicians and education for patients. Within a year of commencing the programme, there was positive improvement. This programme was also carried out with a quality improvement set-up, ensuring the sustainability of the intervention long-term.
Summary
In the authors’ study, the implementation of the EMIS web template improved the screening of PsA in patients with PsO. There was a significant increase in the number of patients with undiagnosed PsA that were detected in the re-audit, once the digital intervention. This has also led to improved awareness of PsA in clinicians, and education for patients. With further repeat PDSA cycles, the opportunistic screening of patients with PsO will lead to earlier and higher detection of PsA in this group of patients.
CONCLUSION
The authors have shown in their study a practical and pragmatic real-world digital approach to improved time to diagnosis in PsA. This is in line with national and international guidelines, and best practice. With earlier diagnosis and screening, this will reduce the physical, functional, social, and financial burden of PsA, which is significant if left untreated. Interventions such as this are needed, as the incidence of PsA is likely to increase in the future. The integrated and interactive approach between all sectors in healthcare will be crucial to achieving the aim of improving outcomes for patients with PsA.







