The pharmaceutical industry could have been better prepared for the COVID-19 pandemic. What did they learn that will help them respond better in the future?
Words by Isabel O’Brien
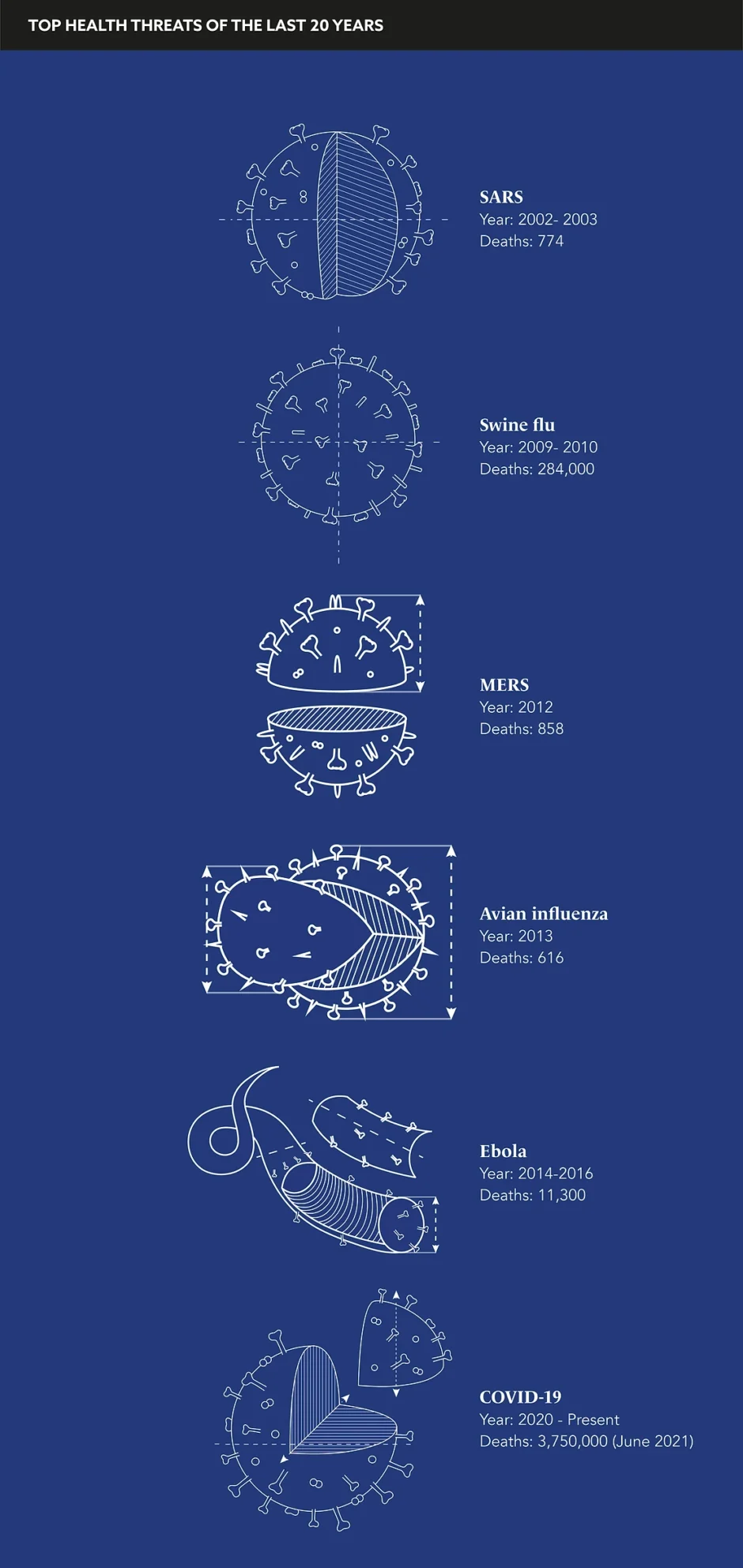
Over the last 20 years, the world has dodged five significant health threats. The outbreaks of SARS, MERS, Ebola, avian influenza, and swine flu were mercifully contained, but the threat of another invasive pathogen has long been both looming and likely.
In fact, Bill Gates has been sounding the alarm since 2014, most notably urging the world to prepare for an outbreak in a now nearly 50-million-time-viewed TedTalk Youtube video.
While hindsight is a wonderful thing, why did the world not respond to the rumbling on the horizon, and crucially, what action can the pharmaceutical industry take now to be ready for the next global health crisis?
“This past year has brought into sharp focus the fragility of life and the complexity of human health,” says Joanna Shields, CEO, BenevolentAI, speaking at the WIREDHealth virtual event. “The COVID-19 pandemic has exposed fundamental flaws in our health systems, supply chains, and disease surveillance, and it has laid bare stark inequalities in society as we continue to face the most significant health challenge of our time.”
The pharma industry, by its own admission, was not ready to tackle a coronavirus outbreak of this pace and magnitude. “At the end of 2019, we had technology in place allowing us to make 10,000 doses of vaccines,” explains Uğur Şahin, Co-Founder and CEO, BioNTech, at WIREDHealth. “By 2020, we needed to exchange that number for the whole planet – for the whole of mankind.”

Mariana Mazzucato, Professor, Economics of Innovation and Public Value, University College London, argues that the transformation of the past year needed not have been so substantial if it were not for our society’s tendency to favour reactive rather than proactive policy making. “The COVID moment is an extremely challenging one in terms of what we have learned about our unpreparedness,” she says at WIREDHealth.
Also the author of Mission Economy: A Moonshot Guide to Changing Capitalism’, Mazzucato continues: “Governments in particular are always thinking that their role is to patch things up; to wait for a crisis before justifying any sort of policy. It is seen as fixing a market failure. If policy and the public sector is there at best to fix market failures, we will always be too little too late.”
It would be dangerous to consider this pandemic a ‘black swan’ event
While there are inescapable trip wires within a culture that relies on reactivity, if we consider previous health threats that have infiltered our society, the patch-up approach has proved to be effective. The outbreak of SARS in 2002 was contained through contact tracing. The swine flu scare of 2009 was curbed by adapting pre-existing influenza anti-virals.
Even Ebola, which statistically is a far more deadly virus, is much easier to identify and contain due to the visibility of its symptoms. However, COVID-19 was unique due to its potential to spread rapidly without detection. We have been lucky in the past, but now times must change if we are to prevent a future crisis of this scale.
While the pharma industry has acted with formidable speed and commendable vigour, it must ponder how it can become more proactive in the realm of contagious diseases. “It would be dangerous to consider this pandemic a ‘black swan’ event,” confirms Shields. “We must use this opportunity to develop advanced technologies that prevent disease, discover new treatments, and protect human health at scale.”
Throughout the pandemic there has been unmatched collaboration between governments, health organisations, and individual pharma companies to fund and accelerate the development of COVID-19 vaccines. Looking forward, Mazzucato believes that the industry must expand these collaborations further and advises that we look to NASA as a role model.
When planning the 1969 moon landing, the space organisation strived to create mutually beneficial public-private partnerships with their collaborators. “They cared about designing the social contract between public and private so that it was goal-oriented – incentivising constant innovation – but also fair in terms of how the profits were distributed,” she says.
The pharma sector has for too long lagged in this arena, and we cannot risk stifling the innovation of treatments that could prevent another serious outbreak. “In the US, $40 billion a year is spent by the government on health innovation, and somehow we don’t have the intellectual property rights to reflect that,” reveals Mazzucato. “We should be thinking about things like a patent pool… how we govern innovation really matters.”
The expanded use of artificial intelligence has been invaluable during this health crisis, not only for identifying high efficacy mRNA vaccine candidates but also for sifting through masses of real-world evidence relating to treatments and treatment combinations to combat the virus. “While the world was erecting barriers, the scientific community was dismantling them,” explains Shields. “To defeat this virus and future pathogens, we need to open communication channels between scientists in every country.”
She warns: “Old models of competition and secrecy will not cut it… to bridge the gap, partnerships between AI companies and pharma are essential.” Given that data is the lifeblood of AI, pharma companies must share their insights so that both clinical decision-making and drug discovery can become more efficient and successful.
We must prepare for the next global health emergency now
Regulatory approval did initially stagnate at the start of the pandemic, but new avenues were quickly opened to expedite urgent submissions. Şahin emphasises the value of converting these emergency measures into permanent, well-oiled solutions. “We’ve had collaborative efforts not only with the companies, but also with institutions and regulatory authorities,” he says. “An amazing factor contributing to our success was that when we transferred our documents for clinical trials, the feedback came in within a few days.”
Overhauling the approval process will enable the industry to accelerate the innovation cycle, prevent the emergence of new pathogens, and rapidly contain future outbreaks that appear.
While pandemics are largely unpredictable events, the industry must be careful to prepare for all threats, ones that are present and ones that are a possibility. “We’ve been living through one of the most challenging times in history,” concludes Shields. “Our ambition is great and our purpose is clear – we must prepare for the next global health emergency now.”
While the industry has demonstrated its razor-sharp reactions, now is the time to devise an action plan so we are prepared long before the rumbling of the next alarm.