Currently, there is a lack of tissue-engineered solutions for replacement of urological tissues, like ureters and the urethra and corpus spongiosum (CS). The bottlenecks to these solutions are vascularisation and the complex tubular organisation composed of different cell layers. The CS is an integral part of the urethra and is important in supporting its function. In cases of a healthy CS, success rates of replacement with epithelial tissue, like oral mucosa, can reach up to 90%. However, in cases of severe fibrosis or the absence of CS in congenital disease (hypospadias), tissue engineering of the urethra should be combined with reconstruction of the CS.1 In a series of presentations at European Association of Urology (EAU) Congress 2018, the use of tissue-engineered oral mucosa grafts (TEOMG) for urethral reconstruction was discussed. In one study, TEOMG reduced the rate of donor morbidity and was successful in around 67% of cases.2 Success rates were dependent on the number of prior surgeries: the more prior surgeries the higher the risk of recurrence of stricture. In cases of failure, the CS may be affected by severe fibrosis due to previous intervention and these patients may benefit from our innovative casting approach to engineer a three-layered tubular construct to mimic the organisation of the native CS.
As part of our poster (poster 285), we presented the CS as a multilayered, highly vascularised structure with distinct distribution of extracellular matrix components (Figure 1A). A mould with three chambers, representing the three layers of the CS, was designed and fabricated. The chambers were loaded with gelatine-based hydrogels containing a coculture of endothelial cells and pericytes (chambers 1 and 3) and smooth muscle cells (chamber 2) (Figure 1B). A fibre mesh was placed at the base of the construct to serve as a porous support for the gels and to roll the construct into a multilayered tubular construct. The gels were mechanically tested and compared to native CS and could easily be rolled into multilayered tubular constructs. The encapsulated cells formed small capillary-like structures (chambers 1 and 3) and produced elastin (chamber 2) within 2 weeks of culturing. The compressive modulus of the construct was comparable to that of the native tissue.
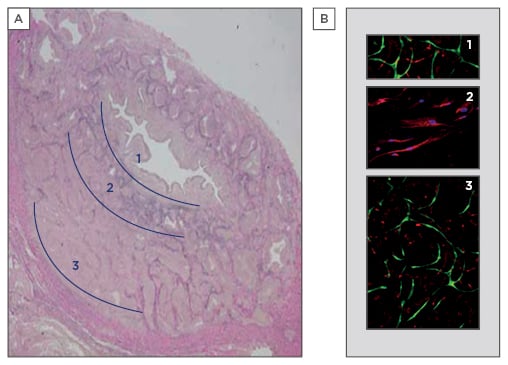
Figure 1: Engineering of a multilayered graft.
A) The three layers of the human corpus spongiosum can be distinguished: 1) rich in microvessels; 2) elastin-rich; and 3) rich in vascular spaces and microvessels (3 μm tissue section, elastin staining in purple). B) Representation of the mould with three chambers, chambers 1 and 3 with endothelial cells (green) and pericytes (red), and chamber 2 with smooth muscle cells (nuclei in blue, elastin staining in red).
In conclusion, our approach enables construction of tubular structures with distinct composition in the different cell layers. Cell survival and functionality of up to 14 days was achieved. Our next steps will involve upscaling to clinically relevant sample sizes and in parallel testing in laboratory animals to assess whether the vascular networks produced in the hydrogel will adapt to the native vasculature. Our tissue-engineered CS can be combined with future applications of TEOMG, and this approach towards tissue engineering of multilayered tubular structures may be applicable to the urological field, as well as in other fields of soft tissue engineering.








