Session Summary
A Foundation for Survivorship With Immuno-oncology and Potential New Approaches for Patients With ROS1+ Non-small Cell Lung Cancer
Jürgen Wolf set the scene, providing an overview of the advancements in available therapies for patients with cancer over the past two decades, highlighting I-O and targeted agents as key pillars in modern treatment approaches in oncology. In terms of NSCLC specifically, he outlined how the landscape has evolved rapidly to include several I-O options for patients in the non-metastatic and metastatic settings, as well as targeted treatments, specifically ROS1 TKIs (Figure 1).1,2
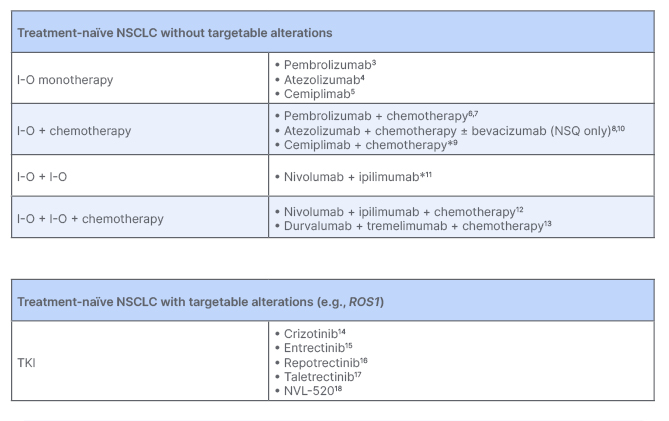
Figure 1: Potential first-line treatment options for metastatic non-small cell lung cancer as discussed in the symposium.
*Approvals/recommendations per PD-L1 status vary regionally.
I-O: immuno-oncology; NSCLC: non-small cell lung cancer; NSQ: non-squamous; PD-L1: programmed cell death ligand 1; TKI: tyrosine kinase inhibitor.
Providing an overview of the potential first-line (1L) treatment options for metastatic NSCLC (mNSCLC), Wolf highlighted the multitude of available I-O treatment options for patients without driver mutations, including I-O monotherapy, and I-O based combinations. He noted that there is currently one validated biomarker, programmed cell death ligand 1 (PD-L1), of which expression can help guide treatment selection for patients who are eligible to receive I-O regimens. For patients with ROS1 fusions, several treatment options are now recommended.1,2
Immuno-oncology Monotherapy for First-Line Metastatic Non-small Cell Lung Cancer
Focusing on recent developments in 1L mNSCLC, Wolf noted the availability of long-term follow-up data from key Phase III trials of I-O monotherapy in patients with tumour PD-L1 ≥50%. He highlighted data from KEYNOTE-024, a study of pembrolizumab versus chemotherapy, in which the benefit of pembrolizumab treatment was maintained over time with a hazard ratio (HR) of 0.62 (95% confidence interval [CI]: 0.48–0.81) after a median of 59.9 months of follow-up.3 Wolf also highlighted the long-term efficacy observed in the EMPOWER-Lung1 and IMpower110 trials, where cemiplimab and atezolizumab continued to show a trend in overall survival benefit versus chemotherapy over a median follow-up of 37.1 months and 31.3 months, respectively.4,5
Immuno-oncology Plus Chemotherapy for First-Line Metastic Non-small Cell Lung Cancer
Turning to I-O plus chemotherapy combinations, Wolf discussed long-term readouts from the KEYNOTE-189 and KEYNOTE-407 trials, in which pembrolizumab plus chemotherapy was compared against placebo plus chemotherapy in patients with non-squamous and squamous mNSCLC, respectively. In both trials, patients who received pembrolizumab plus chemotherapy continued to demonstrate overall survival (OS) benefit over a median follow-up of approximately 5 years.6,7 A long-term survival benefit was also shown with atezolizumab plus chemotherapy versus chemotherapy alone in patients with non-squamous disease (IMpower130),8 and cemiplimab plus chemotherapy versus placebo plus chemotherapy (EMPOWER-Lung3).9 Additionally, in the IMpower150 trial, where patients were treated with atezolizumab plus bevacizumab plus chemotherapy, or bevacizumab plus chemotherapy, the atezolizumab combination was favoured with regard to long-term survival following a minimum follow-up of 32.4 months.10
Wolf highlighted, however, that in the subpopulation of patients with PD-L1 <1%, these combinations did not confer the same long-term survival benefit as was observed in the overall trial populations. Similar 5-year landmark rates were observed with pembrolizumab plus chemotherapy and placebo plus chemotherapy. Other I-O plus chemotherapy combinations had HR CIs that crossed unity, indicating that increased PD-L1 expression may be a potential enrichment factor for these regimens.6-10
Immuno-oncology Plus Immuno-oncology With or Without Chemotherapy for First-Line Metastatic Non-small Cell Lung Cancer
Moving on to I-O plus I-O combinations, starting with the CheckMate 227 trial of nivolumab plus ipilimumab versus chemotherapy, Wolf explained that patients who received nivolumab plus ipilimumab continued to show a durable survival benefit after a median follow-up of 78.8 months, regardless of PD-L1 expression. Within the PD-L1 ≥1% population, the 6-year OS rate was 22% in the nivolumab plus ipilimumab arm compared with 13% in the chemotherapy arm. Similarly, in patients with PD-L1 <1%, 6-year OS rates were 16% in the nivolumab plus ipilimumab arm, and 5% in the chemotherapy arm.11
In addition to I-O plus I-O, the option of adding chemotherapy to this combination in the 1L setting has also been investigated in several studies. Wolf drew attention to the CheckMate 9LA study, in which nivolumab plus ipilimumab plus two cycles of chemotherapy was compared with four cycles of chemotherapy alone. Patients who received combination therapy demonstrated a durable survival benefit after a median follow-up of 54.5 months, regardless of PD-L1 expression. In patients with PD-L1 ≥1%, 4-year OS rates were 21% versus 16% for combination therapy versus chemotherapy alone. In patients with PD-L1 <1%, 4-year OS rates were 23% versus 13% for combination therapy versus chemotherapy alone, respectively.12
Additional data presented included those from the Phase III POSEIDON trial, in which durvalumab plus tremelimumab plus chemotherapy was compared against chemotherapy alone. Patients who received the combination therapy experienced a sustained survival benefit compared with those who received chemotherapy alone (HR: 0.75; 95% CI: 0.63–0.88) after a median follow-up of 46.5 months.13
Wolf closed this section on I-O combinations by highlighting that the observed safety profile across these trials was as expected, with treatments being generally well tolerated. He also mentioned that clinicians are increasingly able to address concerns about tolerability as they gain more experience with using these combinations.1-11 He concluded that despite the evolution of the treatment landscape, many patients still do not benefit from treatment with I-O or I-O combinations, and there remains a need for clinical trials that include tailored approaches and appropriate control arms to develop effective personalised treatment strategies for patients.
Clinical Developments in the ROS1+ Treatment Landscape
Switching to ROS1 TKIs, Wolf first highlighted that several therapies for ROS1+ NSCLC have been developed in recent years. He then examined the clinical efficacy of crizotinib and entrectinib in ROS1 TKI-naïve mNSCLC. Both treatments elicited high response rates, translating into a median progression-free survival (PFS) of 19.3 months with crizotinib (median follow-up: 62.6 months; PROFILE 1001 trial) and 15.7 months with entrectinib (median follow-up: 29.1 months; ALKA-372-001, STARTRK-1, and STARTRK-2 trials).14,15 He noted, however, that despite these data, patients are at risk of developing resistance mutations (e.g., the ROS1 G2032R mutation has been identified in patients who were treated with crizotinib or entrectinib), which lead to treatment failure and disease progression.19,20 This indicates an unmet need for patients for more durable activity with ROS1 TKIs. Wolf suggested that next-generation TKIs, such as repotrectinib, may help address this unmet need.
Wolf presented data from the TRIDENT-1 trial of repotrectinib in TKI-naïve and pre-treated NSCLC. In the TKI-naïve setting, patients who were treated with repotrectinib achieved a median PFS of 35.7 months, and the median duration of response was 34.1 months (median follow-up: 24.0 months). In addition, treatment with repotrectinib led to an intracranial response rate of 89%. Importantly, no TKI-naïve patients who progressed on repotrectinib developed an on-target resistance mutation.16
In the TKI-pre-treated population, patients who received lorlatinib post-crizotinib achieved a median PFS of 8.5 months, while those who received repotrectinib post-ROS1 TKI had a median PFS of 9.0 months (median follow-up of 21.1 months and 24.0 months, respectively).16,22 Of note, Wolf highlighted that among the patients who were treated with lorlatinib, no response was observed in those with a ROS1 G2032R mutation, whereas responses were observed in 59% of patients who were treated with repotrectinib in the TRIDENT-1 trial.16,22 The safety profile of ROS1 TKIs was considered manageable, with <10% of patients experiencing adverse events leading to treatment discontinuation across trials.14–16,19–22 Looking toward the future, Wolf mentioned two additional treatments that are currently under investigation, taletrectinib and NVL-520.17,18
Wolf closed the section by thanking the researchers and patients who helped drive this evolution in the treatment landscape, emphasising the important role of patient advocacy groups in clinical research, adding: “We are now doing research not only for the patients, but with the patients.”
Multidisciplinary Approach to Managing the Expanding Options with Immuno-oncology in Non-metastatic Non-small Cell Lung Cancer
Jose Luis Campo-Cañaveral de la Cruz introduced the session, switching focus from the metastatic to non-metastatic landscape, first drawing attention to the fact that 2- and 5-year survival rates correlate with disease stage, even when the disease is non-metastatic, where patients with Stage IA1 disease have the best outcomes.23 To address the issue of worsening outcomes across disease stage, it is necessary to focus on how patients with non-metastatic NSCLC (nmNSCLC or resectable NSCLC) are treated, and how outcomes can be improved across the spectrum of Stage IA1–IIIC disease. He then shared an overview of the treatment landscape in resectable NSCLC, and the potential role of I-O in the neoadjuvant, peri-operative, and adjuvant settings.
Latest Data on Immuno-oncology in the Neoadjuvant Setting
Campo-Cañaveral de la Cruz described the CheckMate 816 trial, highlighting that it is the first and only Phase III trial in which I-O (nivolumab) plus chemotherapy is being evaluated in the neoadjuvant setting. Patients with resectable Stage IB (≥4 cm)–IIIA NSCLC were randomised to receive either nivolumab plus chemotherapy, or chemotherapy alone prior to surgery, which was followed by optional adjuvant chemotherapy with or without radiotherapy.24
Investigators reported a significant improvement in pathological complete response (pCR; OR: 13.94; 99% CI: 3.49–55.75; p<0.0001) and event-free survival (EFS) with nivolumab plus chemotherapy compared with chemotherapy alone (not reached [NR] versus 21.1 months; HR: 0.68; 95% CI: 0.49–0.93 at the first pre-specified analysis). Notably, patients who achieved a pCR with nivolumab plus chemotherapy experienced a long-term EFS benefit compared with those who did not (HR: 0.13; 95% CI: 0.05–0.37).24
Results from further subanalyses demonstrated that a greater magnitude of benefit was observed with nivolumab plus chemotherapy versus chemotherapy alone in patients with tumour PD-L1 ≥1% compared with those with tumour PD-L1 <1% (PD-L1 ≥1% EFS: HR: 0.41; 95% CI: 0.24–0.70; PD-L1 <1% EFS: HR: 0.85; 95% CI: 0.54–1.32). Campo-Cañaveral de la Cruz concluded that while OS data are not yet mature, there is a trend toward sustained OS improvement in patients who were treated with nivolumab plus chemotherapy compared with those treated with chemotherapy alone.24
The Surgeon’s Role in Resectable Non-small Cell Lung Cancer
Given the positive data on I-O in the neoadjuvant setting, Campo-Cañaveral de la Cruz switched his focus to the role of a surgeon throughout the patient journey, from diagnosis to post-surgical follow-up. He highlighted the key time points of pathological staging and treatment planning, as well as of the surgery itself, and mentioned the following key considerations for surgeons and the multidisciplinary team:
• Resectability: Can the tumour be removed with a complete resection?
• Operability: Is the patient fit to undergo surgery?
• Surgical complexity: How difficult will the surgery be?
• Patient preference: Is the patient willing to undergo surgery?
Campo-Cañaveral de la Cruz noted the importance of collaboration within the MDT, and working with pulmonologists and cardiologists to assess the complexity of the surgery, and how greatly it will impact the patient in terms of surgical recovery. He also emphasised how crucial it is to have proactive and open conversations with the patient, to ensure that they are involved in decision-making processes, and are aware of the potential risks and recovery associated with surgery.
Regarding factors that might impact the potential success of resection, Campo-Cañaveral de la Cruz highlighted that while the field is gradually moving toward a consensus on defining complexity in the neoadjuvant setting, it is still an ongoing process (Figure 2).25,26 He proceeded to revisit the CheckMate 816 trial, and discuss the impact of neoadjuvant nivolumab plus chemotherapy on resection as well.
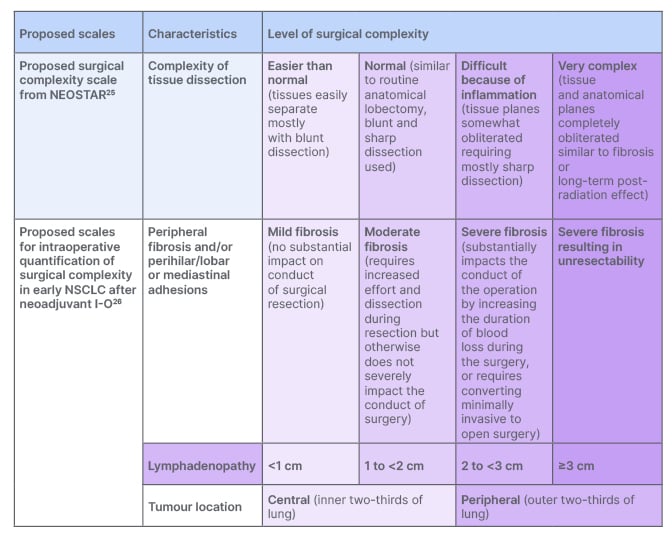
Figure 2: Proposal for the definition of surgical complexity in the neoadjuvant setting.
I-O: immuno-oncology; NSCLC: non-small cell lung cancer.
In the CheckMate 816 trial, the addition of nivolumab to chemotherapy did not impede surgical feasibility.24 Among patients who received neoadjuvant nivolumab plus chemotherapy, 83% underwent surgical resection compared with 75% who received neoadjuvant chemotherapy alone.24 In addition, patients who received neoadjuvant nivolumab plus chemotherapy were more likely to receive a lobectomy and minimally invasive surgery, and less likely to receive a pneumectomy or thoracotomy or progress on their minimally invasive surgery to open, compared with those who received neoadjuvant chemotherapy alone.24 Overall, there were no differences between treatment groups in terms of the timing of surgery or length of hospital stay, and the safety profiles were manageable and similar.24
Latest Data on Immuno-oncology in the Adjuvant Setting
Switching from the neoadjuvant to adjuvant treatment setting, Tina Cascone provided an overview of the latest data from the IMpower010 and KEYNOTE-091 trials. In the IMpower010 trial, patients with Stage IB–IIIA NSCLC who had undergone a complete surgical resection, and subsequently received up to four cycles of chemotherapy, were randomised to receive atezolizumab monotherapy or best supportive care. The primary endpoint in the trial was disease-free survival (DFS);27 patients who received atezolizumab monotherapy experienced an improvement in DFS compared with those who received best supportive care, with the largest benefit being observed in the PD-L1 ≥50% population, excluding patients with known EGFR/ALK mutations (HR: 0.43; 95% CI: 0.26–0.71).27
In the KEYNOTE-091 trial, patients with Stage IB–IIIA NSCLC who had undergone a complete resection and subsequently received optional chemotherapy were randomised to receive pembrolizumab monotherapy or placebo. The primary endpoint in the trial was DFS.28 Patients who received pembrolizumab demonstrated a significant improvement in DFS compared with those who received placebo (median DFS: 53.6 months versus 42.0 months, respectively; HR: 0.76; 95% CI: 0.63–0.91; p=0.0014); however, this benefit was not observed in a subanalysis of the PD-L1 ≥50% population.28 Cascone highlighted that while this was an unexpected result for this subpopulation, the data are not yet mature, and future updates will be important. The safety profiles were also similar across trials, with approximately 20% of patients in both trials experiencing an adverse event, leading to treatment discontinuation.27,28
Utilising Immuno-oncology in the Peri-operative Setting
Moving on to a different treatment paradigm, Cascone discussed several trials in which I-O was utilised in the peri-operative setting: AEGEAN, KEYNOTE-671, and NEOTORCH. Each trial included patients with newly-diagnosed or previously untreated Stage II–III NSCLC.29–31
Patients in the AEGEAN trial were randomised to receive either durvalumab plus chemotherapy or placebo plus chemotherapy prior to surgical resection, followed by durvalumab monotherapy or placebo. The primary endpoints were pCR and EFS; patients who received durvalumab-based treatment demonstrated a significantly improvement in pCR rate (difference in pCR: 13.0%; 95% CI: 8.7–17.6; p=0.000036) and EFS versus those who received placebo (NR versus 25.9 months, respectively; HR: 0.68; 95% CI: 0.53–0.88; p=0.003902).29
Patients in the KEYNOTE-671 trial were randomised to receive either pembrolizumab plus chemotherapy or placebo plus chemotherapy prior to surgical resection, followed by pembrolizumab monotherapy or placebo. The primary endpoints were EFS and OS. Peri-operative treatment with pembrolizumab significantly improved EFS (median EFS: NR versus 17.0 months; HR: 0.58; 95% CI: 0.46–0.72; p<0.00001) and OS (median OS: NR versus 45.5 months; HR: 0.73; 95% CI: 0.54–0.99; p=0.02124) in patients with resectable Stage II–IIIB (N2) NSCLC compared with those who received placebo.30
In the NEOTORCH trial, performed exclusively in China, patients were randomised to receive either toripalimab plus chemotherapy or placebo plus chemotherapy prior to surgical resection, followed by toripalimab plus chemotherapy, or placebo plus chemotherapy, for one cycle, and maintenance toripalimab or placebo for up to 13 cycles. The primary endpoints were EFS and major pathologic response (MPR). Peri-operative treatment with toripalimab plus chemotherapy significantly improved both MPR rate (difference in MPR rate: 40.2%; 95% CI: 32.2–48.1; p<0.0001) and EFS versus placebo plus chemotherapy (median EFS: NR versus 15.1 months; HR: 0.40; 95% CI: 0.28–0.57; p<0.0001).31 Cascone highlighted the importance of these data for patients with Stage III NSCLC as they may be at high risk of relapse. She also mentioned that as observed in the CheckMate 816 trial, patients with a pCR experienced a greater EFS benefit compared with those without a pCR in both the KEYNOTE-671 and NEOTORCH trials.24,30,31
It was noted that surgical outcomes and safety profiles were broadly similar to each other, and in line with expectations across the three trials.24,30,31 Cascone concluded this section by asking an open question: “How can we understand which patients may benefit from each treatment approach, and when should we consider management strategies such as switching, stopping, or amending the treatment regimen?”
Next, she focused on two trials of nivolumab, NADIM II and CheckMate 77T, where it was used in the peri-operative setting. In the Phase II NADIM II trial, patients with Stage III resectable NSCLC received neoadjuvant nivolumab plus chemotherapy followed by surgery. Patients in the nivolumab plus chemotherapy arm who underwent R0 resection subsequently received adjuvant nivolumab monotherapy for 6 months. The primary endpoint was pCR, and key secondary endpoints included PFS and OS at 24 months. Patients who were treated with nivolumab plus chemotherapy showed improvements in PFS (HR: 0.47; 95% CI: 0.25–0.88) and OS (HR: 0.43; 95% CI: 0.19–0.98), compared with those who were treated with chemotherapy alone.32,33
In the Phase III CheckMate 77T trial, patients with previously untreated, resectable Stage II–IIIB NSCLC were randomised to receive either neoadjuvant nivolumab plus chemotherapy or placebo plus chemotherapy followed by surgery and adjuvant nivolumab monotherapy or placebo. The primary endpoint was EFS, and a key secondary endpoint was pCR. Peri-operative treatment with nivolumab plus chemotherapy significantly improved EFS compared with chemotherapy alone (median EFS: NR versus 18.4 months, respectively; HR: 0.58; 97.36% CI: 0.42–0.81; p=0.00025). In addition, a higher proportion of patients experienced a pCR with nivolumab plus chemotherapy versus chemotherapy alone (25.3% versus 4.7%, respectively; OR: 6.64; 95% CI: 3.40–12.97).33 Cascone summarised these data by highlighting that CheckMate 77T is the first peri-operative study to build on neoadjuvant standard of care, and may be a future treatment option.
Key Considerations When Optimising Immuno-oncology Treatment for Non-small Cell Lung Cancer
Cascone closed the session by reviewing the evidence presented regarding the benefits of peri-operative treatment for patients with resectable NSCLC. Looking to the future, she highlighted a few key considerations to help guide the use of I-O in the neoadjuvant or adjuvant settings: Which patients would benefit from neoadjuvant or adjuvant I-O treatments? Which patients would benefit from the addition of adjuvant I-O after receiving neoadjuvant I-O + chemotherapy? Which biomarker(s) can be used to reliably predict response to, or the long-term clinical benefits of, I-O treatments in the peri-operative setting?
Closing with a final point on the role of the MDT in the management of patients with NSCLC, Cascone emphasised the importance of close collaboration and communication between healthcare providers that include, but are not limited to, surgeons, medical oncologists, and pulmonologists, to ensure that patients receive the optimal treatment at the right time to improve outcomes.







