Abstract
Hidradenitis suppurativa/acne inversa is a debilitating, chronic, relapsing inflammatory condition associated with the development of painful nodules and abscesses progressing to persistently draining sinus tracts. This has a great impact on the psychological aspects of the patient and thus on quality of life. The aetiopathological concepts have vastly evolved with time, as have treatment options. Even with the advancement in management strategies, hidradenitis suppurativa remains a formidable problem for the clinician. A collaborative approach management involving the dermatologist and plastic surgeon is mandatory to achieve the most advantageous outcome. Here, a review of recent literature is presented, including a comparison between various management strategies.
INTRODUCTION
In comparison with other cutaneous conditions, hidradenitis suppurativa (HS) has been labelled a lower priority disease for decades. It has been treated by many different specialities, such as plastic surgeons, emergency medicine, family physicians, and dermatologists. This previously orphaned disease has seen an increase in interest in the last few years.1
Lesions are located in the apocrine gland-bearing regions (axilla, inframammary region, groin, and genital area). Disease can develop in any folds of skin, such as the popliteal, but also elsewhere, like the occipital scalp, for example. There are often severe complications in aggravated cases.2 The prevalence of HS ranges from 0.05–4%, and the condition is observed approximately three-times more often in women than in men, with onset between 20 and 24 years of age. The onset usually develops well after puberty in young adults;3,4 however, 5% of cases affect children, with the youngest reported case 5 years of age.5 In children HS is usually associated with family history of HS and/or hormonal imbalance, in contrast to most adult cases.6,7 The definition of HS as presented in the San Francisco odification of the Dessau criteria8 reads:
a) Typical, painful nodular lesions which are located deep within the skin. In early stages, the lesions appear as nodules which develop into abscesses with draining sinuses and bridging scars as the disease progresses. In secondary lesions they might be paired with multiheaded open pseudocomedones.
b) Usual topography is within the apocrine gland-bearing regions.
c) It is a chronic and relapsing disease.
Disease severity is measured with the help of Hurley stages,9 which are often useful to initiate appropriate therapy. Stage I: Abscess formation (single or multiple) without sinus tracts and cicatrisation; Stage II: One or more widely separated recurrent abscesses with tract formation and scars; Stage III: Multiple interconnected tracts and abscesses throughout an entire area.
The psychological impact of HS strongly affects the patient’s quality of life in comparison with other inflammatory skin conditions.10,11
PATHOPHYSIOLOGY
The pathogenesis of HS is not fully understood, but genetic factors, smoking, and metabolic syndrome are known to be associated with HS.12 Histopathological findings demonstrate that the defect is located in the hair follicles, not primarily in apocrine glands. The sequence of the disease development is as follows; initially, infundibular hyperkeratosis appears leading to follicular dilatation accompanied by the formation of cysts. The next stage is characterised by massive inflammation and a local, vivid immune response. This is followed by the fistula, formed of epidermal strands. Early histological examination shows that apocrine sweat glands are not primarily or selectively inflamed.13 It appears that HS, therefore, is a misnomer.12
Immunogenetics of HS has demonstrated the loss of function in three out of four subunits of gamma secretase.14 Potential target proteins are Type I integral membrane proteins, like Notch, E-cadherin, or CD44. Altered Notch signalling is linked with forming defective follicular keratine-enriched epidermal cysts and T cells as dedicated immune response. It is a feedback inhibitor of activated innate immunity. In summary, inherited or acquired altered Notch signalling could be considered a main factor that contributes to the development of HS.15
It is possible that the deficiency in the follicular skin immune response plays a role in the microbial overgrowth associated with HS, but other hypotheses have identified the overactive immune response to normal flora as a possible cause of HS.16 Recently, Ring et al.17 expressed the view that bacterial biofilms have an influence on the chronic lesions of HS and believe HS to be reminiscent of well-known biofilm infections. The results from this study suggested that biofilm formation is associated with the inflammation of chronic HS lesions. Biofilm-driven diseases, in turn, are characterised by chronic relapsing lesions reminiscent of infections recalcitrant to antibiotic treatment.17
Among other influential factors, tobacco smoke seems to play a vital role in the development of HS. Of the 4,000 chemicals within tobacco smoke, those such as nicotine and other particles, activate keratinocytes, fibroblasts, and immunocytes which, in turn, lead to acanthosis, infundibular epithelial hyperplasia, and cornification in keratinocytes. The chemicals present in tobacco smoke induce proinflammatory cytokines, e.g., tumour necrosis factor (TNF)-α and interleukins (IL), leading to neutrophil chemotaxis and T helper 17 cell induction. Nicotine provokes virulence of Staphylococcus aureus16 by inducing biofilm formation. There are many other negative processes caused by cigarette smoke, making the follicle more susceptible to bacterial infections. Additionally, downregulation of Notch ligands, receptors, as well as downstream effector genes, suggest that smoke further suppresses the already deficient Notch signalling in HS.13 Hormones are also thought to play a role in the development of HS, and the disease usually develops well after puberty. In paediatric cases, adrenal hyperplasia, premature adrenarche, obesity, and metabolic syndrome were reported.7 Diagnosing HS in childhood may be a marker of precocious puberty.6
Cases of adrenal hyperplasia in both sexes, including in women with polycystic ovaries suffering from HS, have been reported. There are indications that antiandrogens, such as cyproterone acetate and oestrogens, help in HS, while progestogens induce or worsen a pre-existing HS due to their androgenic properties.18,19 Obesity and metabolic syndrome are also recognised to be strongly associated with HS. The mechanical forces of friction (skin-to-skin and skin-to-clothing) increase follicular occlusion and rupture. In obese individuals, both the mechanism of activating mechanoresponsive genes and the proinflammatory state are present, which play a role in the development of HS.12 Danby et al.20 report that the so-called Western diet can negatively affect people with altered follicular pilosebaceous unit (FPSU). Dairy products have three components that drive the process of FPSU blockage; casein induces the already elevated levels of insulin-like growth factor, while whey and simple carbohydrates raise insulin levels. Acting together or alone, insulin and insulin-like growth factor-1 stimulation depresses the androgen receptor from testes, ovaries, and stressed andrenals, as well as the effects of the FPSU, various contraceptive hormones, and anabolic supplements. In addition to the classical triggers of the FPSU function is 5α-reduced dihydrotestosterone, which is present in the human premenstrual ovary, and at least five others are present in cow’s milk (products of bovine placenta and mammary glands). Reducing weight, and possibly withdrawing dairy products and whey, will break the vicious cycle and correct the chronic hyperinsulinaemia, insulin resistance, and all the related downstream problems, such as hyperglycaemia and hypoglycaemia.20
Currently, the role of the inflammatory response and the cytokines involved in producing inflammation in HS is being investigated. The key role is attributed to TNF-α, a proinflammatory cytokine, produced by both innate and adaptive immune cells in several autoinflammatory conditions. When HS is treated with inhibitors of TNF-α, such as infliximab or adalimumab, an improvement is usually seen. There are several other agents investigated in the HS pathogenesis, such as IL-1β, IL-10, and IL-17.21-24
The entire process of HS development can be summarised as follows: aberrant keratinocytes react with the commensal microbiome, resulting in an altered cytokine and AMP production. The altered production attracts immune cells and triggers an inflammatory cascade; in turn, hyperplastic epithelium causes follicular occlusion and cyst formation. Smoking and false Notch signalling contribute to this process. Cyst rupture allows bacteria and keratin into the dermis, producing a neutrophilic foreign-body type immune response. Finally, abscesses develop, which lead to formation of sinus tracts. Sinus tracts seem to be the HS symptom most resistant to treatment.12 HS is accompanied by comorbidities such as autoimmune disease, inflammatory disease, and skin cancers developing from non-healing wounds.25,26
INVESTIGATIONS
Even though diagnosis is made by clinical assessment,8 some investigations are useful in severe cases. If purulent exudate appears, a microbiological (swab) test may be needed. Imaging using ultrasound and magnetic resonance prove useful in assessing the extent of the disease. Routine blood tests might display potential comorbidities and, in an advanced disease (mainly in Hurley Stage III), may show anaemia or hypoalbuminaemia. Polyclonal hypergammaglobulinaemia, elevated C-reactive protein, and other less commonly used inflammatory markers can serve as an additional factor in monitoring severe cases.
MANAGEMENT
In January 2017, a panel of experts worked out the “European S1 guideline for the treatment of hidradenitis suppurative/acne inversa” (Figure 1),8 stating that the condition should be treated according to individual assessment. Firstly, Hurley stage should be assessed before a treatment plan for the a patient can be established. The experts advise treating localised recurrent lesions surgically, using classic or laser surgery. When the disease is widespread, the favoured approach is to apply an algorithm, either as monotherapy or combined with surgical procedures.8
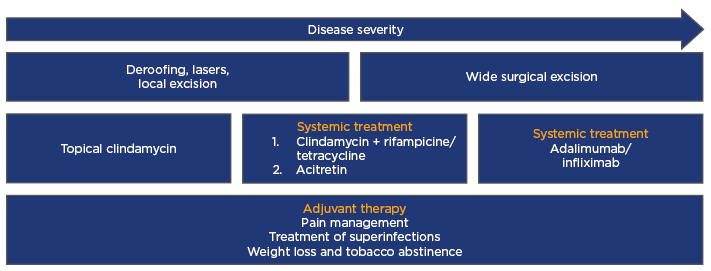
Figure 1: European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa.
Modified from Zouboulis et al.8
MEDICAL MANAGEMENT
Medical therapy can start from skin exfoliants and peels, such as resorcinol.27 A recent study showed that the use of 15% resorcinol in a group of 12 women suffering from HS reported an improvement compared to the prior use of antibiotics and surgery. Topical clindamycin was tested in double-blinded randomised trials. The most significant effect was observed on superficial lesions, in contrast to deep nodules of abscesses, which did not react positively to the treatment.28
The use of systemic antibiotics (such as tetracycline) is advised for HS in Hurley Stage I or II (mild stages). The combination of clindamycin and rifampicin is helpful in more aggravated stages. Antiandrogens proved to be helpful in some cases of HS. Anti-inflammatory therapy plays an important role in the treatment of HS. Intralesional triamcinolone acetonide, as well as a short course of systemic steroids, may be considered prior to surgical management.29
Improvement in patients with HS was also achieved using anti-inflammatories such as dapsone or ciclosporin A. Anti TNF-α biologic medications are also very promising for the treatment of HS. Current evidence shows that adalimumab and infliximab prove helpful in HS treatment, with adalimumab being more tolerable. This may not bring about a complete elimination of the disease but is useful as a neoadjuvant with surgery to decrease the extent of surgery required. Other TNF-α inhibitors have also been studied.30 Isotretinoin and acitretin were also administered to patients with HS but exhibited limited improvement.8,29
SURGICAL MANAGEMENT
Surgical management offers the lowest recurrence rates among all modalities of management for extensive cases. An outcome suggestion so as to have a standard to compare future studies has been put forward by Janse et al.31 The various modalities of surgical management include: incision and drainage of abscess, deroofing, carbon dioxide (CO2) laser, skin-tissue-sparing excision with electrosurgical peeling (STEEP), and excision followed by direct closure, skin stretching system closure, split skin grafting, split skin grafting plus VAC, local flaps, and free flaps.
Incision and Drainage
The simplest of all surgical procedures performed on HS is incision and drainage of an acute abscess. This is only a temporary measure to manage the collection of pus; relapse is inevitable because the root cause is not treated.
Deroofing
This is a procedure by which the extent of the lesion is assessed with a probe and the roof, along with the jelly-like material on the floor of the sinus tracts, are removed by electrosurgical dissection. In a study on 88 deroofed lesions,32 the recurrence rate was 17%, indicating this is effective as a minimally invasive technique for surgical management. Advantages include a simple, minimally invasive technique, and disadvantages include not that all of the diseased tissue is dealt with, leading to a high risk of recurrence.
Carbon Dioxide Laser
The sinuses are explored with blunt forceps and then deroofed by evaporation using CO2 laser.33,34 The slough at the bottom of the tracts are also cleaned up with CO2 laser and left to heal by secondary intention. Advantages include that it can be performed under local anaesthetic and offers an effective method. Disadvantages include that the procedure requires costly equipment and trained personnel.
Skin Tissue Saving Excision with Electrosurgical Peeling
Described by Blok et al.,35 this procedure, performed under general anaesthetic, involves initial deroofing of the lesions followed by tangential electrosurgical transection of the involved deeper layers until the whole area is clear of lesional tissue and fibrosis. The wounds are left open to heal by secondary intention. Advantages of STEEP include that there is a more extensive and complete removal of diseased tissue than deroofing technique, spares healthy tissue, and avoids more extensive procedures like grafts and flaps, and expensive equipment like laser. Disadvantages include that completion of excision of diseased areas may not be thorough, leading to higher recurrence rates than with more extensive surgical procedures, and prolonged healing time from leaving open to heal by secondary intension and thus more scarring results.
Complete Surgical Excision
This technique is resorted to only after medical management has failed to keep the disease under control. Once the excision has been achieved, there are various options to get the wound to heal. The method selected depends on the extent of area involved, anatomical site, and surgeon’s preference.
Leave Open to Heal by Secondary Intension
Following surgical excision of the involved area, the wound is left to heal by granulation, contraction, and epithelialisation. Secondary intention healing is recommended as a safe and efficient form of healing of postoperative defects in certain areas of the body.36 This technique avoids the morbidity associated with donor site healing as in grafts and flaps; however, a long duration is required for complete healing and is limited in terms of size of wound.
Direct Closure
This is applicable only for small areas of involvement, as enough normal skin should be available for a tension-free closure after complete excision of the diseased area. In a study of 92 local excisions with primary closure, 66% had no recurrence.37 This is usually feasible in Hurley Stages I and II. Direct closure offers the best cosmetic result with minimal postoperative healing problems and because it is usually used for small areas, it can be performed under local anaesthetic. However, it can only be used if the area of involvement is small.
Sure Closure: Skin Stretching System
This technique, described by Sharma et al.,38 uses the viscoelastic properties of the skin, so as to stretch the skin by exerting a controlled amount of tension along the wound margins. Known as the skin stretching system, this technique facilitates primary closure of a wound that would have otherwise required either graft or flap. Skin stretching allows closure of a larger wound than that can usually be primarily closed, and the bedside procedure can be performed under local anaesthetic. Although it does give a higher propensity for hypertrophic scar formation, infection of intradermal pin tracts and ischaemic necrosis of skin edges can occur if stretching is not done with caution.
Split Skin Grafting
If the raw area resulting from excision is too large for a primary closure, the options are either graft or flap. A split skin graft is used, which can be harvested from almost any area as it heals by itself and the graft can be meshed to cover wider areas. Due to it being very thin, the graft moulds well to the contour of the recipient site. Split skin graft, however, does not transfer sweat glands and hair follicles to the recipient site and thus reduces the risk of recurrence at the site postoperatively.39 The contracture associated with split skin grafts is acceptable in non-flexural areas, like the gluteal region.
Split skin grafting can be combined with negative pressure dressing or vacuum assisted closure.40 This method overcomes the difficulties associated with immobilising the grafted area to achieve a good ‘take’ of the graft postoperatively. A sponge dressing is applied over the non-adherent dressing on the graft and a controlled negative pressure applied allowing adherence of the graft conforming to the contour of the site, preventing shearing, and is also beneficial in drainage of collections between the graft and bed.
Any size defect can be resurfaced in a single procedure and the thin graft moulds to the contour of the recipient site avoiding a bulky appearance. Risk of recurrence on the operated site is low. There is, however, need to have immobilisation of the operated area until the graft is taken up, which usually takes a week. In flexural surfaces, a splint is required to prevent contracture as the graft undergoes secondary contraction after healing and the cosmetic appearance is compromised.
Flaps
Extensive areas of involvement requiring excision leaves raw areas that need tissue from elsewhere. A flap provides similar tissue with a similar texture and nature as the normal skin of the area. Simple flaps from the adjacent area as either a transposition, advancement, or rotation are commonly used. A transposition flap can be a fasciocutaneous flap, such as a lateral thoracic fasciocutaneous flap.41 This flap is based on perforators supplying the skin of this region from the lateral thoracic and thoracodorsal arteries. It is a robust flap, large defects can be covered using this flap and it can be raised as an island flap based on the vessels, in which case it is possible to thin the flap to the required thickness.
A thoracic artery perforator V-Y flap42 is based on the musculocutaneous perforators of the thoracodorsal vessels that supply the skin over the muscle. This can be marked with a hand-held Doppler in the theatre and flap raised based on the perforator position (Figure 2). Compared to a random supply, the V-Y flap43 allows free movement of the flap into the recipient site. A double V-Y technique,44 which is perforator based, is helpful if a larger defect needs coverage. Here, one flap from the chest wall and the other from the arm are used in a double opposing manner. A parascapular perforating flap45 is an option available locally, which provides greater mobility to the flap but involves more tedious dissection.
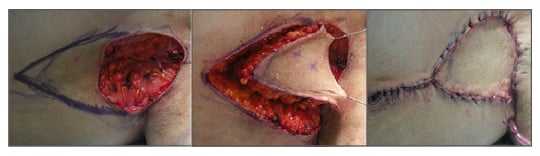
Figure 2: Thoracic artery perforator V-Y flap.
A Limberg flap (Figure 3) has been described in a case series by O’Brien et al.46 and Altmann et al.,47 for coverage of areas of HS involving the axilla. A recent review of Limberg flaps for the same condition has substantiated its usefulness in this situation.48 Gluteal, perianal, and perineal areas of involvement can similarly be treated with simple fasciocutaneous49 transposition, rotation, or V-Y flaps. The perianal area is more challenging to reconstruct, especially when the defect is extensive. The extended split superior gluteus maximus musclocutaneous flap described by Kishi et al.50 is a very good option. Diverting colostomy51 is indicated occasionally in patients with involvement of these areas undergoing surgery. Medial thigh lift following radical excision of hidradenitis of the groin results in a cosmetically pleasing appearance. A good functional and aesthetic result has been reported in a series of 15 thigh lifts by Rieger et al.52 Modified abdominoplasty53 can be used for HS involving the lower abdomen and groin region, or a combination of abdominal flap and thigh lift54 if reconstruction of a more extensive area is required.
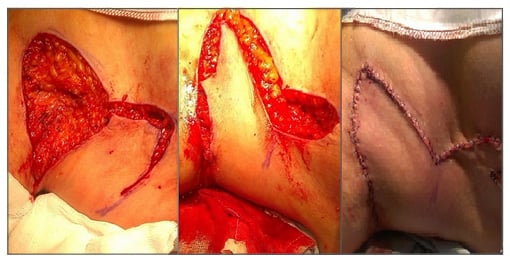
Figure 3: Limberg flap coverage of areas of hidradenitis suppurativa involving the axilla.
HS involving gluteal, intergluteal, and perianal areas are usually treated with deroofing, leaving to heal by secondary intention or skin grafts. Superior and inferior gluteal artery perforator flaps55 are locally available flaps that can be designed for even large defects in this area to facilitate immediate closure, providing excellent quality tissue and good cosmetic appearance in terms of not only the reconstructed area but also the donor site scar. The anterolateral thigh flap56 and pedicled gracilis myocutaneous flap57 are other options available for the difficult groin and perineal areas. Advantages of flaps include good colour and texture match, and early mobilisation is possible, unlike grafts; however, they do require more surgical expertise than grafts and could result in a bulky appearance and donor site scars.
A recent systemic review on recurrence rates after surgical management showed the following results: wide excision (13.0%), local incision (22.0%), and deroofing (27.0%). In the wide excision group, recurrence rates were 15% for primary closure, 8% for using flaps, and 6% for grafting.58 A 10-year study by Kagan et al.59 of 57 patients involving different areas such as the axilla, inguinal, and perineal region showed no recurrence.
Another relevant report in literature regarding recurrence rates following complete excision is by Balik et al.60 involving 21 surgeries in 15 patients. Wounds were left open, primarily closed, or skin grafted. A 5-year follow-up showed no recurrence and they concluded that recurrences were due to incomplete excisions. Incomplete excision would inevitably result in a recurrence. Having an array of reconstructive options in the armamentarium gives the surgeon the freedom to avoid limited excisions and to do complete excisions of all disease bearing areas that could result in absolutely no recurrence in the treated area.







