Abstract
The process of successfully weaning patients from invasive mechanical ventilation is a great challenge for all healthcare providers working in critical care. Despite several recent advances in the care of intensive care patients, failed extubation remains a significant problem that may result in poor patient outcomes. A lack of consensus in many areas regarding clinical approach to extubation and the peri-extubation period exists, and the numerous strategies described in this review add to the complexity of the decision faced by the clinicians involved.
The process of weaning and timing of extubation may be improved by implementation of a consistent multidisciplinary approach to weaning, with a number of easily identifiable risk factors available to support clinical decision making. There are also many known risk factors that can be used to predict the likelihood of extubation failure; whilst these factors may not be easily modifiable, they do allow the identification of patients at a high risk of extubation failure who may require more detailed care and planning post extubation. Finally, a number of strategies, including non-invasive ventilation and high flow nasal oxygen therapy, are available to support carefully selected groups in the post extubation period. Evidence is emerging linking these adjuncts to a reduction in the risk of extubation failure. This article will discuss these risk factors and the evidence supporting their use in this challenging patient group.
INTRODUCTION
The process of weaning from invasive mechanical ventilation (IMV), including discontinuation of IMV and removal of the endotracheal tube or tracheostomy from a patient’s airway, is an integral step in the management of a patient in the intensive care unit (ICU). Guidance on the optimal strategies and timing of weaning are varied and the process is beset by potential complications. Extubation failure is arguably the most serious complication of weaning and is defined as the need for reintubation within a 48-hour period of initial removal of the patient from IMV.1 Extubation failure is associated with several adverse healthcare-related outcomes and is thus of great significance to both healthcare providers and patients.
Despite numerous advances in intensive care management in recent years, extubation failure rates have remained relatively unchanged over the last decade, with ≤25% of patients extubated in an ICU requiring reintubation within 48 hours.2 It is widely reported that reintubation secondary to post extubation respiratory failure is associated with several adverse outcomes, including increased hospital length of stay and mortality.3,4 These risks, however, must be balanced against the risks associated with continued and prolonged IMV, which, in turn, may be associated with significant complications.5
Several approaches have been studied to improve the process of weaning from IMV by facilitating a successful transition from IMV to spontaneous ventilation. These range from careful patient selection to supportive treatments to minimise the risk of failure following extubation. This article will review the current evidence behind these strategies and discuss the challenges encountered when extubating patients in the ICU.
WHEN TO EXTUBATE
It is not disputed that in the acute setting, both intubation and IMV are essential as well as life-saving interventions that are mainstays of care in critically ill patients.6 However, it is also well documented that prolonged IMV is associated with a number of adverse outcomes, including ventilator associated pneumonia, ICU-acquired weakness, and increased hospital length of stay.5,7 Several stages of mechanical ventilation have been proposed (Figure 1), with the weaning process beginning after reversal of the precipitating cause that led to intubation.1 Weaning encompasses the patient journey from the initiation of reducing ventilator support to the removal of the endotracheal tube.8
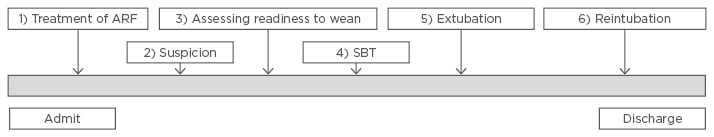
Figure 1: Stages of mechanical ventilation.
ARF: acute respiratory failure; SBT: spontaneous breathing trial.
Adapted from Boles et al.1
This transition from IMV to spontaneous breathing in the critical care population is often complex with multiple confounding factors to consider9 and differs significantly from the process of waking and extubation following general anaesthesia for surgical procedures. Patients starting the weaning process early and extubating rapidly have lower rates of mortality and morbidity.5 Despite awareness of this, weaning from IMV in the ICU is often delayed, which increases the risk of patient complications as well as the cost and stress on already stretched healthcare resources.1,5,10 The rationale that weaning can often be started earlier in the ICU is supported by the findings of Epstein et al.,11 who found that ≤50% of patients accidentally extubated in the ICU did not require subsequent reintubation.
Despite the presence of several studies comparing different methods of weaning, no real consensus as to the optimal weaning method exists. Some authors promote gradual reduction in pressure support (PS)12 over a period of time until patients reach a point of being comfortable on little or no PS; other studies suggest that daily spontaneous breathing trials (SBT) (often via a T-piece) are just as effective.13,14 Perhaps of greatest importance when faced with such a challenging clinical problem is a multi-disciplinary team approach. The inclusion of nursing staff, physiotherapists, and specialists in the rehabilitation process to help deliver care, giving different perspectives, is thought to have a beneficial effect on clinical outcomes in weaning patients.15 Thorough assessment, consistency in practice, adherence to principles of best practice, established care bundles such as ventilator-associated pneumonia prevention, and consideration of each patient’s individual care, are all vital elements when planning a weaning strategy.9
Liberation from mechanical ventilation via the process of extubation is arguably the pivotal event within the weaning process and must be performed at an early enough stage to limit the risks of prolonged IMV but not so early as to increase the risk of extubation failure and all its associated complications. As such, it is vital that clinicians consider a large amount of clinical and patient factors prior to making the decision to proceed to extubation. This decision involves an evaluation of the patients’ ability to breath spontaneously and consideration of other factors, such as how and when to extubate, the potential for airway difficulty in the event of extubation failure, and what interventions may be available to prevent life threatening complications. Despite principally pertaining to anaesthesia practice, it would be naïve of the intensivist to disregard the Difficult Airway Society16 guidance on extubation, which is relevant to critical care practice. This guidance highlights the issues that may be easily overlooked, including assessing for previous airway difficulty and optimisation of non-patient factors, including location, the presence of skilled personnel, and the availability of emergency airway equipment, such as airway exchange catheters and laryngeal mask airways. Patient position is also important, with semi upright positions being preferred to facilitate spontaneous breathing and relative ease of reintubation if required.16,17 The 4th National Audit Project (NAP4) of the Royal College of Anaesthetists18 highlighted critical care as a high-risk area for adverse airway events, with poor identification of at-risk patients, insufficient planning, and inadequate skilled personnel reported as triggers of such events. It is therefore paramount that those involved in the extubation process are fully aware of the potential pitfalls of airway management and are trained accordingly.16
In contemporary critical care practice, there has been a move away from performing early tracheostomy to facilitate faster weaning, based on the findings of several large and robust studies,19,20 and as such it is becoming less common for the procedure to be performed in patients ventilated for <10 days. However, there remains a cohort of critical care patients, including those with known upper airway obstruction or neurological conditions resulting in lasting bulbar dysfunction, in whom an early tracheostomy without attempted extubation may be beneficial. This prevents patients in whom successful extubation is highly unlikely being exposed to the often significant risks associated with a failed extubation.
Measures of respiratory muscle strength and capacity, such as the rapid shallow breathing index (RSBI) and maximal inspiratory pressure, correlate inconsistently with a patient’s ability to successfully extubate. Additionally, individual clinician judgement has been demonstrated to have a low sensitivity and specificity in predicting success.5 Thus, the currently favoured approach is to conduct a SBT lasting between 30 minutes and 2 hours and assess the patient’s clinical response during this period. During a SBT the patient breathes with little or no inspiratory PS, using 5–8 cmH20 PS or a T-piece attached to the ventilator circuit.6 Again, no clear consensus exists regarding the most appropriate mode or duration of SBT;6 however, it has been suggested that application of no additional inspiratory PS yields greater validity in the SBT due to its more realistic resemblance of spontaneous breathing. Furthermore, studies have demonstrated that most SBT failures occur within 30 minutes,21,22 suggesting that a successful SBT of 30 minutes is as good an indicator of successful extubation as one of 120 minutes. Failure of an SBT is commonly defined by the presence of worsening physiological parameters, such as tachycardia, tachypnoea, hypertension, agitation, and anxiety,8 or a deterioration in gas exchange or ventilatory parameters,12,13 and should be followed by a period of patient stabilisation on increased ventilator support before a further SBT is considered.
A recent systematic review conducted by the American College of Chest Physicians (ACCP) and American Thoracic Society (ATS),6 concluded that, for patients ventilated for >24 hours, an SBT with PS of 5–8 cmH20, rather than a T-piece trial, increased the success of the SBT and subsequent extubation and thus may represent a reasonable approach to assessing readiness to extubate.
PREDICTORS OF SUCCESS AND FAILURE
Despite robust patient assessment with daily sedation holds and SBT, extubation failure remains a significant problem, with reintubation rates remaining as high as 25%.23 This implies that a SBT alone is not the sole consideration when making the decision to extubate, and it is equally important to attempt to identify patients at a high risk of extubation failure prior to discontinuing IMV. Unfortunately, this remains a difficult clinical decision, with no one test available to perfectly recreate post extubation conditions.3,4 Clinicians must endeavour to balance the risk of delayed versus premature extubation, both of which are associated with significant increases in poor outcomes,24 and attempt to use as much information as they can relating to the risk of failed extubation in their patients to guide decision making.25
Many of the historical studies investigating extubation failure have been small single-centre trials of low-risk individuals, which lessens the validity of their findings when applied to the wider ICU population.3 A 2006 study4 exploring risk factors for failed extubation following a successful SBT revealed an extubation failure rate of 13.4% for those passing an SBT, which was in concordance with previously documented figures. Work of breathing, hypoxia, respiratory acidosis, retained secretions, reduced conscious level, and hypotension were all reported as risk factors for reintubation in this study. Although this is noteworthy and useful information, there remains the issue of how modifiable or preventable such risk factors are in clinical practice and to what degree these problems may be treatable prior to extubation failure occurring.
A positive fluid balance is widely recognised to have a detrimental effect on extubation success; a finding supported by studies suggesting that congestive cardiac failure patients have higher reintubation rates.26-28 Furthermore, positive fluid balance is associated with longer periods of IMV29 and, therefore, may compound the increased risk of extubation failure with the increased morbidity and mortality that occurs secondary to prolonged mechanical ventilation. B-type natriuretic peptide levels have recently been associated with weaning duration. Elevated B-type natriuretic peptide levels at SBT may predict extubation failure30 and thus could potentially be used to rule out cardiac dysfunction as a source of weaning and extubation failure.
Upper airway obstruction, or oedema, is also an important cause of extubation failure, especially in those patients who successfully pass an SBT and appear to have adequate respiratory mechanics, with one study quoting airway patency as a direct cause of extubation failure in ≤38% of patients.31 In the presence of a negative cuff leak test, steroid administration may reduce the prevalence of stridor and reduce the rate of reintubation32 and, thus, should be considered in all patients undergoing extubation with clinical signs of upper airway oedema. The duration of suggested steroid regimes in the literature varies, although a large meta-analysis of 14 studies33 suggested that steroids should be administered ≥12 hours prior to extubation and for ≤24 hours after. Should concerns exist regarding airway patency without an endotracheal tube or tracheostomy in situ, extubation should not be attempted and the opinion of an ear, nose, and throat surgeon should be sought.
The RSBI has been extensively investigated in relation to failed extubation, with several studies supporting its reliability as a predictor of failed extubation.34 One study concluded that an RSBI >105 was an independent risk factor for failed extubation, with reintubation rates increasing from 11% to 18%.4 Thus, it seems reasonable to measure RSBI, where possible, prior to making decisions regarding extubation of patients, but it is also vital to consider that while RSBI may be a reliable indicator of respiratory muscle capacity, it does not make any assessment of the airway problems highlighted above and should not be used in isolation as a marker of suitability for extubation.
Risk factors resistant to modification, such as age, pneumonia (as the cause for mechanical ventilation), conscious level, cardiovascular disease, and respiratory disease,35,36 may not be parameters that can be influenced or changed by clinical care but remain important factors in clinical decision making. Recognition of these factors allows early identification of patients at a high risk of extubation failure and thus enables clinicians to dedicate more attention and resources to patients in the extubation period to achieve the best possible patient outcomes.
Other risk factors less confidently associated with extubation failure include cough strength, secretion load, the presence of delirium, and ICU-acquired polyneuromyopathy. These factors have limited and somewhat conflicting evidence of association, with further studies necessary to define their predictive value in extubation failure.37,38 A summary of the risk factors for extubation failure and the strength of supporting evidence are shown in Figure 2.

Figure 2: Risk factors for extubation failure following invasive mechanical ventilation.
ICU: intensive care unit; IMV: invasive mechanical ventilation; RSBI: rapid shallow breathing index.
HIGH-RISK PATIENTS AND NON-INVASIVE VENTILATION
Having identified those at a high risk of extubation failure, measures must be taken to avert complications in the post extubation period. Non-invasive ventilation (NIV) may be used to provide respiratory support without the need for tracheal intubation in a wide range of recently extubated patients.39 This may be in the form of continuous positive airway pressure or non-invasive positive pressure ventilation. Its use in the post extubation period falls into three distinct patient groups that will be addressed separately; namely, as an aid to early extubation, as a prophylactic measure in high-risk extubations, and finally as a treatment for post extubation respiratory distress.40
Early extubation and progression to NIV for continued weaning in patients who have failed an SBT but are suitable for weaning has been demonstrated to reduce the length of IMV time and risks associated with ongoing IMV in selected patient groups.41 This effect appears to be most prominent in patients with chronic obstructive pulmonary disease (COPD) and has been replicated in several randomised controlled trials and meta-analyses.42,43 A large multicentre study by Girault et al.44 that recruited from a more general population of ICU patients found no significant difference in reintubation rates between patients extubated early onto NIV versus conventional weaning, with time spent in the weaning phase increased in the NIV group. It has been suggested that the use of NIV in this way is not presently recommended for general ICU populations but may be an appropriate course of action in patients with chronic respiratory disease, which is supported by recent guidance from the British Thoracic Society (BTS) as a strategy for weaning patients with known COPD.45
Prophylactic use of NIV post extubation has been extensively studied in patients deemed to be at a high risk of extubation failure. This has been demonstrated in a number of studies to significantly reduce reintubation rates when used before the onset of post extubation respiratory distress.23,46,47 The ACCP, ATS,6 and BTS45 all present comparable advice stating that patients receiving >24 hours of mechanical ventilation who are at a high risk of extubation failure should receive prophylactic NIV immediately post extubation. Although some debate exists as to what actually constitutes a high risk of extubation failure,48 with several different definitions used in different studies, there is a general consensus on a number of factors that warrant prophylactic post extubation NIV: being a smoker, age >65 years, known respiratory or cardiovascular disease, poor cough.23
NIV in acute respiratory failure caused by pathologies such as exacerbations of COPD49 and pulmonary oedema50 is an established treatment that can prevent the need for mechanical ventilation. However, the use of NIV in the treatment of post extubation respiratory failure has been shown to be both ineffective and potentially detrimental. It has been suggested that NIV used in this setting may lead to delays in reintubation once respiratory compromise has occurred, which in turn may increase patient morbidity and mortality;51 as such, its use is not supported in this setting.
POST EXTUBATION HIGH-FLOW NASAL OXYGEN THERAPY
Following extubation, the conventional method of preventing hypoxia is application of controlled oxygen therapy (COT), usually via a facemask with the fraction of inspired oxygen targeted to a physiological parameter. Facemask oxygen, however, can be cumbersome and is associated with variable levels of oxygen delivery dependent upon the user’s peak inspiratory flow. In addition, mucosal drying may occur secondary to a lack of humidification,52 increasing the risk of extubation failure secondary to secretion retention. High flow nasal oxygen therapy (HFNOT) is a relatively new development in adult populations offering humidified, warmed oxygen at flow rates ≤60 L/min.53 This may be beneficial to recently extubated patients by providing more accurate oxygen concentrations, generating positive end expiratory pressure and improving gas exchange.54
Several studies have investigated HFNOT use in post extubation ICU populations. Maggiore et al.53 reported improved oxygenation and fewer desaturations compared to COT, perhaps due to improved patient tolerance of the nasal delivery system, and a non-significant trend towards reduced reintubation rates. Hernandez et al.55 concluded that low-risk patients treated with HFNOT had lower reintubation rates and less commonly developed post extubation respiratory failure compared to those treated with COT. A more recent study,56 terminated early due to recruitment issues, found no significant difference in the frequency of post extubation respiratory failure, time to respiratory failure, or length of ICU and hospital stay with HFNOT.
A recently published meta-analysis examining the role of reintubation in post extubation patients suggested that HFNOT is more effective at preventing reintubation than COT57 and may be as effective as NIV in this setting but without the side effects and patient tolerance problems that may hamper effective NIV delivery. It is important to note that one of the larger studies in the data pool excluded patient groups known to respond well to post extubation NIV, such as those with COPD and cardiogenic pulmonary oedema; thus, the impact of NIV may have been underestimated in this trial.
The use of HFNOT in post extubation patients is a promising development, particularly in those patients deemed at a low risk of developing extubation failure or where NIV intolerance may be an issue. The precise role of HFNOT and how it may be used alongside NIV to achieve optimal clinical outcomes requires further clarification, and robust trials are needed in this important area.
CONCLUSION
Despite many recent advances in ICU practice, optimal management of extubation remains a significant challenge to healthcare providers and carries a significant weight of morbidity and mortality should extubation failure occur. Several weaning strategies are well described in the literature, with an organised approach and consistency in practice seemingly more important than the weaning method used. Although a number of factors are described that may predict extubation failure, few of these are easily modifiable and no universal consensus exists to guide clinicians on when exactly to extubate.
A number of interventions are available to support patients who have been recently extubated, and in particular the timely application of NIV may be greatly beneficial, especially in patients with chronic lung disease or when risk factors present for extubation failure. There is also growing interest in the use of HFNOT in lower-risk patients, and this therapy may play a useful role in carefully selected post extubation populations.
It is evident from the literature that careful planning and assessment at every stage of the patient journey through the ICU, from an organised multi-disciplinary team approach to weaning, through to provision of suitable respiratory support following extubation, are essential to achieve the best possible outcomes in this challenging patient group. Further work is warranted to more clearly define and stratify the risk to patients of extubation in the ICU and identify which treatment strategies should be most effectively used to improve patient outcomes in this challenging area of contemporary practice.








