Abstract
Objective: Pharmacovigilance assessment of a novel intrauterine spherical ball-shaped copper intrauterine device (Spherical Copper 300 mm2 intrauterine device [IUD]; OCON Medical Ltd., Modiin, Israel]) using data collected from both users and healthcare providers.
Study Design: Pharmacovigilance reports of undesirable side effects (events) were collected and evaluated based on healthcare providers who completed pharmacovigilance reporting related to device insertion and outcomes.
Results: Data was reviewed from 111,022 insertions from 23 countries. Insertions were performed by multiple types of providers: nurse practitioners, midwives, general practitioners, and obstetrician-gynaecologists. Out of 111,022 insertions, a total of 496 accumulative pregnancies were reported over a period of 70 months, for an effectiveness of 99.6% (n=110,706) over the reported period. Few complications were reported. Of those reported, expulsion rate was 3.30% (n=3,619), with median time from insertion to expulsion of 7 weeks (0–60 weeks), perforation rate of 0.05% (n=60), and pelvic inflammatory disease rate of 0.02% (n=23).
Conclusions: The Spherical Copper 300 mm2 IUD was used in a diverse group of women by a variety of medical providers. Spherical Copper 300 mm2 IUD use suggests high efficacy and safety with infrequent complications. The Spherical Copper 300 mm2 IUD is an additional option for women desiring reliable, reversible, long-acting, and non-hormonal contraception.
Implications: Females interested in a safe and highly effective, long-acting, reversible contraceptive method would benefit from additional options. Currently, the T-shaped Copper 380 mm2 IUD is the most prevalent, non-hormonal, long-acting, reversible contraceptive method for females, with over 99% effectiveness. The Spherical Copper 300 mm2 IUD offers an alternative contraceptive option for females who do not desire exogenous oestrogen or progestin exposure.
INTRODUCTION
Unintended pregnancies1,2 have significant and potentially negative impacts on females and their families. Further, these pregnancies disproportionately affect younger females, as well as those of minority groups and lower socioeconomic sectors.3 Public health agencies support programmes which promote and expand access to long-acting, reversible contraception (LARC); intrauterine devices and implants. These programmes have produced some positive effects in reducing the global incidence of unintended pregnancies.1,4
LARC methods do not require action by the user to maintain efficacy and function for extended periods of time. Additionally, they are as effective as sterilisation (pregnancy rates are less than 1 per 100 female years), but reversible.5 LARC methods, such as the hormonal levonorgestrel intrauterine devices, non-hormonal copper intrauterine devices, and subdermal levonorgestrel or etonogestrel implants, have produced high levels of efficacy with good safety profiles in most female populations, including adolescents.6 Pregnancy rates among IUD users have been shown to range from 0.4–1.4%, depending on the type of device used (copper IUD and levonorgestrel IUD), and the length of use.7-9
This is in contrast to the higher 12-month pregnancy rates of approximately 5–7% and between 9–10% in 3 years in typical use of combined oral contraceptives, due primarily to missed pills or failure to resume therapy after the pill-free interval.4,10 Intrauterine contraception satisfaction is high, with reported 12-month continuation rates ranging from 74–88%.11,12
The Copper 380 mm2 IUD, named for the 380 mm2 of copper surface area, is the most widely used IUD worldwide.13 The Copper 380 mm2 IUD has a plastic T-shaped core with a copper wire and copper sleeves placed around the vertical stem and horizontal arms, respectively. Like many of the IUDs, the frame of the Copper 380 mm2 IUD is connected to a monofilament thread that protrudes through the cervical canal into the upper vagina for easy removal.14 T-shaped IUDs are introduced into the uterine cavity through an inserter measuring between 3.6–4.4 mm in diameter, depending on the type of IUD.15
The International Organization for Standardization (ISO) 7439 is an international standard that specifies requirements and tests for single-use copper IUDs and their insertion instruments, which determines the need between 200–380 mm2 of copper surface area.16 While current copper IUDs are highly effective, those with at least 300 mm2 are most effective.17
The Spherical Copper 300 mm2 IUD facilitates a different design. It is comprised of several copper-eluting beads, with a copper surface area of 300 mm2. The beads are strung on a flexible, polymer-coated, nickel-titanium (nitinol) wire ball-shaped frame pre-loaded, in a linear form, in a narrow insertion tube measuring 3.2 mm in diameter. The memory-shape alloy nitinol is necessary to introduce a 3D sphere into the uterine cavity through the thin cervical canal. The nickel-titanium frame is covered by a protective polyethylene terephthalate coating, and company internal copper and nickel release kinetics determination tests in simulated uterine fluid showed no nickel and titanium ion concentrations were found in any analyses, or it was under the detection limits (10 μg/L and 5 μg/L for titanium and nickel, respectively).
The nitinol technology used for the Spherical Copper 300 mm2 IUD is known from the use in cardiovascular stents. The frame of the Spherical Copper 300 mm2 IUD is corrosion-resistant and super elastic to resist breakage and fragmentation, as reported with traditional plastic T-shaped IUDs (Figure 1). A double-tailed monofilament removal thread is attached to the tip of the frame. The Spherical Copper 300 mm2 IUD was designed to adapt to the geometry of the intrauterine cavity by forming a spherical shape at insertion. This loop-on-loop shape was designed to absorb pressure from uterine contractions that can occur in a normal menstrual cycle. The Spherical Copper 300 mm2 IUD is currently commercially available within certain regions of Europe, the Middle East, and Africa, as a Class III medical device.
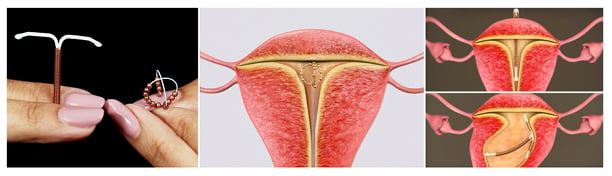
Figure 1: Illustration of the T-shaped versus ball shaped copper intrauterine devices and potential displacements.
The objective of this post-marketing retrospective pharmacovigilance study was to collect data from users and healthcare providers who completed pharmacovigilance reporting. The authors’ primary outcome of interest was undesirable side effects (events), including pregnancy, expulsion, perforation, and other side effects of the Spherical Copper 300 mm2 IUD. The authors hypothesised that the Spherical Copper 300 mm2 IUD would be highly effective, with a low risk of complications, and therefore be an additional safe, long-term, reversible contraceptive option.
METHODS
Analysis of post-marketing, observational, retrospective pharmacovigilance data was performed to assess the safety and efficacy of the IUB® Ballerine® MIDI (OCON Medical Ltd., Jerusalem, Israel) Spherical Copper 300 mm2 IUD on a cohort of diverse females. All devices were inserted by trained medical providers (nurses, midwives, general practitioners, and obstetrician-gynaecologists). After inserting the tube into the uterine cavity, the device is deployed from the distal tip of the inserter tube and retrogradely curled away from the fundus to form a spherical shape composed of four perpendicular semi-circular lobes, forming an anatomically compliant sphere with a diameter of 15 mm (Figure 1).
Data for this report were collected from 23 countries across Europe, the Middle East, and Africa. The data was collected in accordance with the obligatory Post-Market Surveillance (PMS) standards defined by the European Medical Device Regulation and ISO requirements. This data is part of the manufacturers quality management system, which is regularly audited by the Notified Body. Insertions were performed between March 2015–December 2020. Spherical Copper 300 mm2 IUD users were healthy, nulliparous, or multiparous, and were 15 years of age and older. Individual-level data on Spherical Copper 300 mm2 IUD users are not available. Contraindications included pregnancy, uterine cavity distortion, acute pelvic inflammatory disease, endometritis, known uterine or cervical malignancy, genital bleeding of unknown aetiology, untreated acute cervicitis or vaginitis, Wilson’s disease, allergy to any component of the Spherical Copper 300 mm2 IUD, increased susceptibility to pelvic infection, or presence of an IUD.
Pharmacovigilance reports of undesirable side effects (events) by healthcare providers provided the dataset for this analysis. Reports of pregnancies or complications included the following information: patient age, parity, date of insertion, date of event, event description, and information on discontinuation of device. Reports did not include information on females who had no side effects. Reports were collected through local distributors. Pregnancy rate was calculated by dividing the number of pregnancies reported by the number of estimated insertions reported, which represents accumulative pregnancy rates over a period of 70 months (March 2015–December 2020). One difference in this pharmacovigilance data is that healthcare providers were encouraged to report adverse events while receiving free product replacement units for reporting of any event over the 5-year product lifespan. Distributors submitted a quarterly PMS reporting table directly to the manufacturer, OCON, which included the estimated number of insertions per quarter.
The raw follow-up data were cleaned prior to analysis by Premier Research (Morrisville, North Carolina, USA). The data cleaning process involved removing duplicate events that occurred on the same date for the same subject, and implementing various rules to ensure that all useable data can be applied for the analysis. As such, analysis datasets were created from the raw follow-up data that included categories for insertion, pregnancy, expulsion, and perforation.
Analysis flags were derived from the raw data to indicate if an event occurred. These flags were used to calculate the number of times an event occurred, as well as the percent and associated 95% confidence intervals. The 95% confidence interval was obtained by using the means procedure calculated through SAS version 9.4 (SAS Institute, Cary, North Carolina, USA). These analyses were performed for all 23 countries.
RESULTS
Of the 111,022 insertions performed in 23 countries, the highest number was performed in Germany (n=33,796), followed by Spain (n=10,533), while the fewest number of insertions were performed in Uganda (n=11; Table 1). Approximately half (51.4%; 57,081 out of 111,022) of the insertions occurred in countries with at least 5 years of experience inserting the Spherical Copper 300 mm2 IUD (Austria, Germany, Israel, and Switzerland). No differences in outcomes were observed based on length of time the Spherical Copper 300 mm2 IUD had been available.
The rate of insertion failures overall was 0.09% (104 out of 111,022). The range of time for duration of device use by providers is between 0.9–7.4 years, based on launch dates in the different territories.
There were 496 (0.40%) pregnancies reported over a period of 70 months (Table 2). No difference was seen in pregnancy rate by country. The mean age of the females who became pregnant was 27.9 years (16–46 years). Ectopic pregnancies were reported in 10 (0.01%) females.
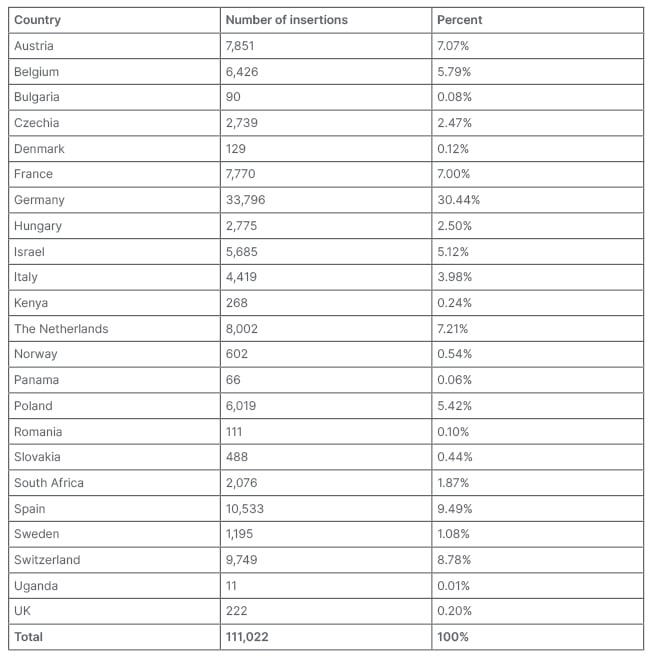
Table 1: Countries and number of insertions.
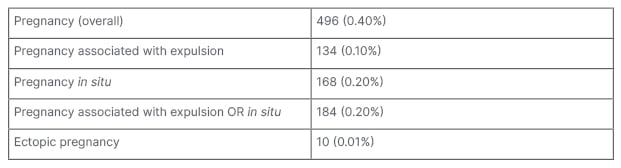
Table 2: Overall efficacy of 111,022 Spherical Copper 300 mm2 intrauterine device insertions.
The number of expulsions reported was 3,619 (3.30%; Table 3). The mean age of the females who had expulsions was 26.4 years (15–49 years). Out of the 3,619 expulsions, 1,391 (3.30%) occurred in females under 25 years of age. The median time to expulsion was 7 weeks after insertion (0–60 weeks; Table 4). Uterine perforation was infrequent, occurring in 60 (0.05%) females.
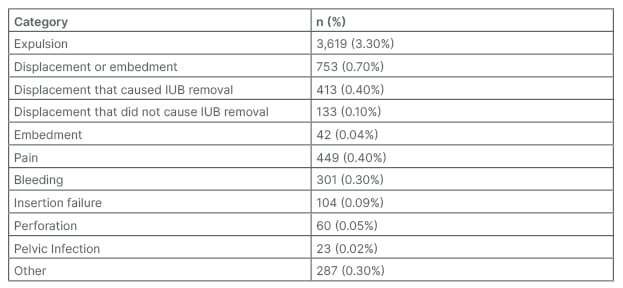
Table 3: Complications of 111,022 Spherical Copper 300 mm2 intrauterine device insertions.
IUB: intrauterine ball.
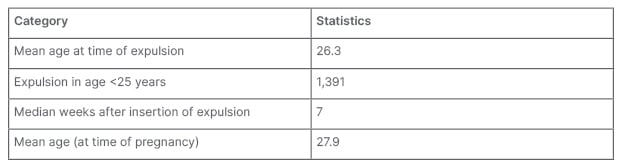
Table 4: Summary of expulsion or pregnancy by age.
The total number of displacements for the Spherical Copper 300 mm2 IUD was 546 females (0.5%). The number of displacements that resulted in Spherical Copper 300 mm2 IUD removal occurred in 413 (0.4%) females; whereas the number of displacements that did not result in Spherical Copper 300 mm2 IUD removal occurred in 133 (0.1%) females. Other complications included embedment in 42 (0.04%) females, pain in 449 (0.40%) females, bleeding in 301 (0.30%) females, and pelvic infection in 23 (0.02%) females.
DISCUSSION
In this large retrospective study of 111,002 insertions, the reported pregnancy rate of Spherical Copper 300 mm2 IUD was low overall, at 0.4%. This is comparable to other IUDs. For instance, Sivin et al.7 reviewed overall pregnancy rates of 1.4% after 5 years in the Copper 380 mm2 IUD group in their 5-year investigation, and an ectopic pregnancy rate of 0.6/1,000.7 Rowe et al.8 found overall pregnancy rates of 0.43% (levonorgestrel 52 mg IUD) and 1.39% (Copper 380 mm2 IUD) groups, respectively, after 3 years, with an ectopic pregnancy rate in the Copper 380 mm2 IUD group of 0.14% after 3 years and 0.25% after 7 years. Another study by Aoun et al.9 found an overall pregnancy rate of 1% after 3 years (levonorgestrel 52 mg IUD and Copper 380 mm2 IUD combined).
The overall reported expulsion rate of the Spherical Copper 300 mm2 IUD in this study was 3,619 (3.3%). This compares to a 6.0% rate found by Aoun et al.,9 including both levonorgestrel 52 mg IUD and the Copper 380 mm2 IUD. The Spherical Copper 300 mm2 IUD 3.3% expulsion rate is lower than the 10.2% overall expulsion rate found by Madden et al.,18 including the levonorgestrel 52 mg IUD and the Copper 380 mm2 IUD. In this study, more expulsions were observed in females aged 14–19 compared with older females, regardless of parity or IUD type.18 Rowe et al.8 found expulsion rates of 5.2% (levonorgestrel 20 mg) and 5.6% (Copper 380 mm2 IUD) after 3 years. Additionally, the study by Sivin et al.7 found 5-year expulsion rates of 11.8% (levonorgestrel 20 mg) and 7.4% (Copper 380 mm2 IUD), respectively.
The Spherical Copper 300 mm2 IUD reported overall perforation rate was 0.05% (0.5 per 1,000 IUB insertions, with 60 total events). Heinemann et al.19 found 1.4 perforations per 1,000 insertions in the levonorgestrel-intrauterine system group and 1.1 perforations per 1,000 insertions in the T-shaped Copper 380 mm2 IUD group in their 1-year follow-up investigation.
Copper-containing IUDs can be associated with heavy menstrual bleeding. For instance, a randomised trial found that long-term Copper 380 mm2 IUD users were more likely than levonorgestrel 20 mg users to discontinue the device because of heavy menstrual bleeding and dysmenorrhoea (9.7 per 100 females versus 1.3 per 100 feamles, respectively), whereas levonorgestrel 20 mg users were more likely to discontinue the device because of amenorrhoea and spotting than Copper 380 mm2 IUD users (4.3 per 100 females versus 0 per 100 females, respectively).20 Although this current study did not measure bleeding profiles, the design of the Spherical Copper 300 mm2 IUD is thought to potentially minimise heavy menstrual bleeding.
One theory why the T-shaped Copper IUD may cause heavy menstrual bleeding relates to misalignment, which may lead to endo- or myometrial trauma, which could increase the risk of malposition and embedment within the uterine tissue.21,22 Among 167 patients with a T-shaped IUD evaluated using the 3D reconstructed coronal view ultrasound, 28 (16.8%) had an IUD with side arms abnormally located within the myometrium. The abnormal positioning of the IUD arms was only detected using the 3D coronal view. A higher proportion of patients with an abnormally located IUD presented with bleeding (35.7%) or pain (39.3%), compared with those with normally positioned IUDs (15.1% with bleeding and 19.4% with pain [p=0.02 and 0.03, respectively]). A total of 75% of patients with an abnormally located IUD presented with bleeding or pain compared with 34.5% of those whose IUD was normally placed (p=0.0001). Out of 21 patients with an abnormally located IUD, 20 presented with pelvic pain or bleeding, and reported improvement in their symptoms after IUD removal.23
This study uses pharmacovigilance data to assess efficacy and safety. An additional example of this type of study was used in a French population-based cohort study. A historical cohort of females aged 20–55 years with a first dispensing of either levonorgestrel 52 IUD (n=11,891) or copper IUD (n=10,194; the copper IUD types included 11 different products) between 1st January 2010–31st December 2014, in the French National Claims database (SNDS), was carried out. It found, over a median follow-up of 3.3 years (interquartile range: 2.3–4.6 years), in the overall population, 0.2% had an ectopic pregnancy, 0.1% had a pelvic inflammatory disease or infection due to IUD, and 0.2% had a uterine perforation due to an IUD. Additionally, 47.0% removed their IUD.24
A cross-analysis comparing the Spherical Copper 300 mm2 IUD results of the real-world PMS data collection with a previously published study conducted in Israel25 shows comparable rates of expulsion and pregnancy. This suggests that the majority of side effects are reported within the PMS, providing supporting evidence for the reliability of these data.
The authors’ data, collected on over 100,000 insertions, indicate that the use of the Spherical Copper 300 mm2 IUD is safe and efficacious. The authors found no unexpected complications. Effectiveness appears to be at least as good as other IUDs that are currently approved for use. The Spherical Copper 300 mm2 IUD is potentially a new, long-acting, reversible, non-hormonal contraceptive option for females.
Study Strengths and Limitations
Strengths of this study include the large number of insertions evaluated (111,022), multiple types of providers, and the large number of recruiting countries. It was done over 5 years (March 2015–December 2020) accumulatively.
The sponsor offered free replacement devices at any time during the product’s lifetime (5 years) for any reason, in order to motivate adverse events reporting.
The Spherical Copper 300 mm2 IUD dataset is based on a pharmacovigilance collection, which potentially has an underreporting limitation compared with a prospective clinical trial. Yet, the authors think the incentivisation to report by receiving free devices minimised this limitation. They authors believe the risk of fraud reporting is minimal due to the requirement to provide detailed information with each reported case.
Limitations of the study are that demographics of the study population and details of the adverse events are limited. Insertions are estimates based on information provided by distributors. No standardised process of collecting information for the adverse events was utilised; however, the data presented represents a largescale number of insertions, with reassuring efficacy rates and low complication rates.
CONCLUSIONS
The Spherical Copper 300 mm2 IUD was an effective birth control option for many nulliparous and multiparous females from 23 countries. Further, the pregnancy rate and complication rates were low. The Spherical Copper 300 mm2 IUD is emerging as a reliable long-acting contraceptive for females.
This article uses the recently published IUD nomenclature released by the World Health Organization (WHO).26







