Meeting Summary
This integrated symposium took place on 17th April 2023 in Copenhagen, Denmark, during the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) 2023. Presenters included Birgit Weinberger, Institute for Biomedical Aging Research, University of Innsbruck, Austria; Tino Schwarz, Institute of Laboratory Medicine and Vaccination Centre, Klinikum Würzburg Mitte, Standort Juliusspital, Germany; and Albert Osterhaus, Centre of Infection Medicine and Zoonosis Research, University of Veterinary Medicine, Hannover, Germany. The aims of the symposium were to describe the burden of infectious diseases in the adult population, including individuals with compromised immune systems resulting from underlying conditions or immunosuppressive therapy; to present factors that increase the risk and severity of clinical outcomes of communicable diseases in adults; and to assess the global status and value of adult immunisation. Prevention options and current gaps in awareness and understanding of infectious diseases were also presented. Weinberger discussed age-specific incidence of infectious diseases and the risk factors for infectious diseases in adults, focusing on the mechanism of immunosenescence and immunosuppression. In this context, Weinberger emphasised the importance of adult immunisation to protect against infectious diseases and reduce overall disease burden. Schwarz summarised the global epidemiology, characteristics, and complications of herpes zoster (HZ), discussed comorbidities and immunosuppressive conditions with increased risk for HZ, and highlighted the importance of prevention of HZ through vaccination. Osterhaus explained how respiratory syncytial virus (RSV) is a threat to adults as well as infants, describing the importance of RSV in respiratory illnesses, and the current diagnosis and treatment of RSV in older adults, and summarised the research conducted to identify the burden of RSV in the adult population. These topics were then discussed in a lively question-and-answer session.
Introduction
Infectious diseases such as influenza, HZ, and RSV infection are a risk to healthy ageing because of immunosenescence, which contributes to a decreased capacity of the immune system to respond effectively to infections or vaccines in the elderly.1,2 Adults with compromised immune systems are at an increased risk of infection and re-infection, with potentially more severe presentations compared with those who are immunocompetent.3 Maintaining the health of an ageing population, particularly for those individuals who are immunocompromised, is a challenge best addressed by considering all aspects of health management, including comprehensive strategies for prevention, diagnosis, and treatment of infectious diseases. Lessons learned from the management of infectious diseases such as influenza, including the utilisation of diagnostic tools and vaccines, are a useful guide for the development of strategies for other infectious diseases, including HZ and RSV infection. An understanding of the burden, awareness, and gaps in management of these infectious diseases in adults at risk is essential to optimise management strategies and patient care.
Infectious Diseases in Adults: Who Is at Risk, Why, and What Can We Do About It?
Birgit Weinberger
Who Is at Risk?
Weinberger explained that many infectious diseases have an age-specific incidence. For example, rates of hospitalisation for influenza are highest in infants aged <1 year and adults aged ≥65 years.4 In contrast, the incidence of HZ starts to increase at approximately 50 years of age, and this is consistent across different countries.5 Age is not the only risk factor for severe infection; comorbidities can also increase risk for hospitalisation.6 The presence of seven or more comorbidities is a very strong indicator for hospitalisation for influenza.6 Even without comorbidities, increasing age is associated with a higher risk for hospitalisation;6 however, the prevalence of comorbidities increases with age.7 In line with this, global mortality associated with comorbidities such as myocardial infarction, stroke, and cancer is greatest in adults aged ≥70 years.7 Patients with immunosuppression are also at increased risk for infectious disease compared with the total population, and age is an additional risk factor.3 Across all adult age groups, patients who have undergone bone marrow or stem cell (8.9-times) or solid organ (3.5-times) transplantation, and those with HIV (3.6-times), are at increased risk for HZ versus the general population.3 Weinberger believes that awareness among physicians is high for these risk groups, but there are also lesser known groups at increased risk for HZ, such as patients with systemic lupus erythematosus (3.2-times) or rheumatoid arthritis (2.5-times), that “may not be on our radar.”3
Why Are They at Risk?
Weinberger emphasised that there are many potential causes for increased incidence and severity of infections with age. These include blunting of the cough and swallow reflexes, reduced cilial motility in the lungs and gastrointestinal tract, physiological changes in the urogenital tract, and structural changes of the skin and mucosa.8,9 All these changes facilitate the entry of pathogens into the body. Older age is also associated with reduced immune function, and lower resilience due to underlying organ dysfunction; therefore, older individuals are more susceptible to infection, and tend to have more severe illness when they become infected. Other external risk factors include hospitalisation, invasive procedures (e.g., catheters), and use of antibiotic medication.8,9
Focusing on reduced immune function, Weinberger described the mechanism of immunosenescence and immunosuppression, why these phenomena are associated with higher risk of infectious diseases, and their poor clinical outcomes in certain adults. Weinberger explained that age-related changes in immune response are numerous, contributing to age-associated diseases and reduced vaccine responses.10 Defects in the cells of the innate immune system in older age include reduced migration and phagocytosis,1 altered cytokine production,1,10 and reduced antigen processing and presentation.1,10 Also important are the changes in the adaptive immune system, including fewer naïve T cells,1,10,11 more effector T cells,11 and functional T cell defects,1,10,11 leading to altered cytokine production.1,10,11 In addition there are fewer naïve B cells,1,11 and defects in B cell isotype switch (a biological mechanism in which B cell production of immunoglobulin changes from one type to another, e.g., from IgM to IgG or IgA) and somatic hypermutation (optimisation of binding affinity of antibodies).1,10,11 In addition to the age-related decline in immunity, immunosuppressive disease conditions, as well as disease conditions requiring intensive immunosuppressive therapies, can disrupt B and T cell-mediated immunity independent of age.10,12 Adults who are immunocompromised are at increased risk of more severe infections and re-infections compared with those who are immunocompetent.3
What Does Burden of Disease Really Mean?
According to Weinberger, the burden of infectious disease often goes well beyond the common definition of acute illness and hospitalisation.13 This is particularly true in patients who are elderly or chronically ill, in whom exacerbation of comorbidities (e.g., chronic obstructive pulmonary disease [COPD]),14 and transient increases in risk of cardiovascular events,15,16 are important consequences of infection. For example, rates of acute myocardial infarction in the first week after influenza diagnosis have been shown to be six-fold higher than 1 year before and 1 year after the risk interval (defined as the first 7 days after respiratory specimen collection and the ‘control interval’; Figure 1).17 Also reported are a 68% elevated risk of acute myocardial infarction in the first week,18 and a 78% increased risk of stroke in the first month, post-HZ.19 Weinberger considered that decline of general health status, increase in frailty,20 and possible loss of functionality and independence,21-23 following severe infection are frequently underappreciated by healthcare professionals and patients. All of these components of disease burden are relevant, and impact individuals, healthcare systems, and societies.24,25
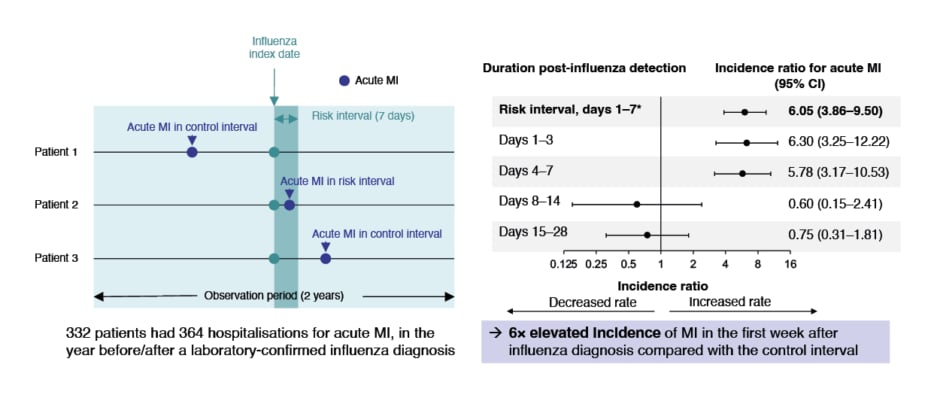
Figure 1: Risk of acute myocardial infarction after influenza infection.
*Primary analysis CI: confidence interval; MI: myocardial infarction.
The same results were first published in Kwong JC et al.;17 the graph has been independently created by GSK from the original data.
What Can We Do?
Weinberger advocated vaccination to protect against infectious diseases and reduce potential disease burden. However, vaccination recommendations for influenza26,27 and pneumococcal infection27-29 for older adults vary across Europe. In addition, Weinberger noted: “Vaccine recommendations can be complicated. If we want to improve availability and uptake of vaccines, it is essential to have straightforward and consistent recommendations.”
Why Do We Not Do It?
Focusing on seasonal influenza vaccination coverage in older adults in Europe, Weinberger observed that only a few countries reached the European Union (EU) target level of 75%, and there was a decrease in coverage between 2007 and 2015 in several countries, including the Netherlands, Italy, Spain, and France.30 Moreover, Weinberger believes that this trend is ongoing. A study of self-reported vaccination history in a European cohort (aged 35–75 years; n=2,100) in 2011 showed that tetanus and diphtheria vaccination rates varied greatly, with the highest rate (83%) in Germany and the lowest (10%) in Greece, and this was reflected in the antibody titres.31 Weinberger stated: “Uptake of vaccines in adults is poor in many European countries, and there is an ongoing trend towards decreased coverage. There is an urgent need to reverse this trend and improve vaccine coverage in at-risk adults, as well as address the heterogeneity in guideline recommendations.”
Herpes Zoster: Burden in At-Risk Populations and Its Prevention
Tino Schwarz
Global Epidemiology and Characteristics of Herpes Zoster
Schwarz expressed that HZ is caused by reactivation of latent varicella zoster virus (VZV) in the neuronal ganglia,32 and is characterised by dermatomal rash with debilitating pain and reduced quality of life.33 Age is the most important risk factor for HZ.34 The risk of HZ infection increases dramatically after 50 years of age (Figure 2)5,35 with over two-thirds of cases occurring in this age group.34 Schwarz emphasised: “Everyone is at risk of HZ, and this is something that we have to communicate to our patients.” Decreased VZV-specific cell-mediated immunity in older or immunocompromised patients leads to VZV reactivation.32 The incidence of HZ has increased in developed countries in recent decades, and is predicted to continue increasing with an ageing population,5 because of immunosenescence in advancing age.36
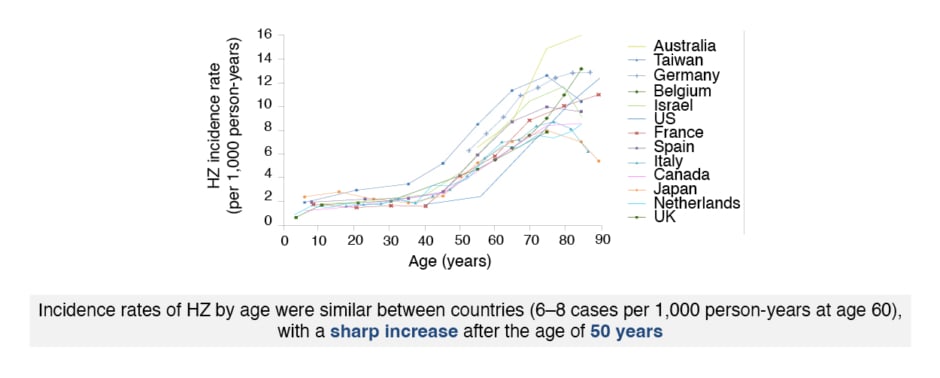
Figure 2: Age-specific incidence rates of herpes zoster infection in North America, Europe, and Asia-Pacific.
HZ: herpes zoster.
The figure has been reproduced with the permission of BMJ Publishing Group Ltd. It was first published in Kawai K et al.5
Complications of Herpes Zoster
The most common and important complication of HZ is post-herpetic neuralgia (PHN),37 defined as neuropathic pain that persists for ≥90 days after the onset of HZ rash,5,38,39 occurring in 5–30% of patients.5 The incidence and duration of PHN increase with age.5,39 Another common complication involves the ophthalmic division of the trigeminal nerve, causing HZ ophthalmicus, in approximately 10–15% of patients.5 Chronic pain in the trigeminal nerve is severe and is a limiting factor. Eye-related complications occur in 30–78% of these patients.5 Corneal involvement, including epithelial keratitis (which can lead to corneal scarring), neurotrophic keratopathy, and stromal keratitis, occurs in 65% of HZ ophthalmicus cases.40 Other ocular complications include conjunctivitis, uveitis, episcleritis, and ocular hypertension.41 Less common are neurological complications, including aseptic meningitis, encephalitis, and myelitis42-44 (<1% of patients with HZ aged ≥50 years),45 and cerebrovascular and cardiovascular complications46 (1% of patients with HZ).47 HZ is an established risk factor for stroke, particularly within 1 month post-HZ rash onset.19 Typically, stroke occurs several months after HZ rash.48,49 Overall, approximately 10% of patients aged ≥50 years with HZ experience at least one non-PHN complication.5 Schwarz emphasised: “Considering the impact on patients of PHN, HZ ophthalmicus, and other complications, HZ prevention is a key element for preserving good quality of life for elderly individuals.”
Comorbidities with Increased Risk for Herpes Zoster
Comorbidities and psychological factors that increase the risk of HZ include asthma,36,50,51 diabetes ,36,52-55 chronic kidney disease36,56,57 cardiovascular disease,36,51,58-61 COPD,36,51,61 depression and psychological stress,36,51,61-64 and physical trauma.36,64-66 The risk of HZ is also increased in patients aged ≥50 years who tested positive for COVID-19.67 Schwarz noted that these comorbidities are commonly encountered in primary care practices; therefore, it is important to educate general practitioners regarding the increased risk of HZ in these patients.
The heightened risk of HZ in patients with COPD36,51,61 is further increased by the use of inhaled or oral corticosteroids.68,69 Schwarz deliberated whether pulmonologists are aware of the effects of corticosteroids on HZ risk, and whether they would recommend HZ vaccination in patients with COPD. Individuals with COPD are at increased risk of developing PHN,70,71 as are those with asthma,72 or diabetes.73 HZ may worsen underlying diabetes.74 Schwarz highlighted that bidirectional links between cardiovascular conditions and HZ have been demonstrated, but this information is not included in medical training, and “we need to rewrite our textbooks.”
Immunosuppressive Conditions with Increased Risk for Herpes Zoster
Patients with immunodeficient conditions36,75,76 and those on immunosuppressive drugs77 are at increased risk of HZ compared with the general population. HIV-related immunosuppression significantly increases HZ risk,36 but the incidence of HZ is substantially lower among patients with HIV who are receiving antiretroviral therapy.78 Schwarz suggested that specialists caring for patients with immunosuppression, including haematologists, rheumatologists, neurologists, oncologists, gastroenterologists, and dermatologists, should be informed of HZ risk and encouraged to prevent HZ in their patients. Guidelines that recommend vaccinations in patients receiving immunosuppressive drugs (JAK inhibitors) include those from the UK79,80 and Spain.81 Although immunocompromised populations represent a small proportion of the total number of HZ cases, they contribute substantially to the public health burden because of their higher risk for HZ75 and HZ complications.3 Symptoms and complications of HZ may be atypical or more severe,77,82,83 and recurrent HZ is more likely,84,85 in patients who are immunocompromised compared with individuals who are immunocompetent.
Herpes Zoster Prevention Through Vaccination
Zoster vaccine live is a live-attenuated vaccine indicated for the prevention of HZ and HZ-related PHN in individuals aged ≥50 years.86 This vaccine is contraindicated in immunocompromised populations.86 Recombinant zoster vaccine (RZV) is a non-live, recombinant, adjuvanted vaccine indicated for prevention of HZ and PHN in adults aged ≥50 years and adults aged ≥18 years at increased risk of HZ.87-90 This vaccine can be administered to individuals who are immunocompromised.88 It is given using a two-dose schedule.86 The adjuvant system for this vaccine boosts the immune response, eliciting a strong, specific, and long-lasting immune response against VZV, thereby targeting the decline in VZV-specific cellular immunity seen in older adults and some immunocompromised populations.88,89 Pooled analysis of pivotal Phase III studies (ZOE-50/70) in patients aged ≥50 years showed sustained vaccine efficacy in patients with various comorbidities.91 RZV has also been shown to be efficacious against HZ in patients who are immunocompromised, including recipients of autologous haematopoietic stem cell transplantation.87,92 Solicited local and systemic adverse events are more common with RZV than placebo,93,94 and Schwarz recommended that patients are informed of the reactogenicity of the vaccine, including the possible development of local pain and redness following vaccination.
Respiratory Syncytial Virus: More Than a Threat to Infants?
Albert Osterhaus
The Importance of Respiratory Syncytial Virus in Respiratory Illnesses
Osterhaus specified that among children aged under 5 years, there are more than 10 million cases of pneumonia95 and 900,000 pneumonia-related deaths96 reported worldwide each year. Pneumonia is a greater worldwide cause of childhood mortality than malaria, tuberculosis, HIV, Zika virus, and Ebola virus combined.97 Osterhaus emphasised that the most common cause of severe pneumonia requiring hospital admission in children aged 5 years or under without HIV infection from Africa and Asia is not bacteria (27%), but viruses (61%), including RSV (31%) and influenza virus (2%).97,98 Osterhaus noted that children usually do not die from influenza, whereas inadequately treated RSV is associated with considerable mortality.99 Virtually all children have experienced at least one RSV infection by 3–5 years of age.100 Furthermore, severe acute respiratory illnesses have been shown to be temporally associated with outbreaks of RSV and influenza in adults, with similar levels and burden of RSV-positive and influenza-virus-positive disease.101
The Current Diagnosis, Treatment, and Vaccination Landscape of Respiratory Syncytial Virus in Older Adults
There is considerable overlap of symptoms for RSV, influenza A, human metapneumovirus (hMPV), rhinovirus/enterovirus, and coronavirus infection in older adults; therefore, these infections cannot be diagnosed based on clinical symptoms alone.102 Osterhaus recommended that clinicians access information on the viruses circulating in their geographical area, and utilise available diagnostic tools, where possible, to identify these infections in their patients. According to Osterhaus, little effort has been made to diagnose RSV infection, except in paediatric hospitals, and most cases of RSV-related pneumonia or other lower respiratory tract infections are not being diagnosed.103 There is currently no vaccine for RSV, and there are no antiviral therapies for RSV infection, with only symptomatic treatment available for older adults.104,105
The Burden of Respiratory Syncytial Virus in Adults
Osterhaus noted that the physical condition of older adults in the Western world has generally improved in the last 40 years; however, immunocompetence in older age has not improved in parallel.100 Human infection challenge studies show divergent age-related humoral correlates of protection against RSV infection, with young adults showing a stronger local immune response and lower viral load in the respiratory tract compared with older adults.106
There has been considerable research on the burden of RSV in adults over the last 20 years. Key research is summarised chronologically by publication date as follows. In a landmark study published in 2005 by Falsey et al.,107 RSV infection was associated with 11.4% of COPD hospital admissions and 7.2% of asthma admissions in patients aged ≥65 years.107 In addition, RSV infection developed annually in 3–7% of healthy elderly patients and 4–10% of high-risk adults.107 Osterhaus noted that RSV infection is largely considered by physicians as a disease of babies, infants, and children.107,108 However, these results indicate the significant impact of RSV infection in older adults.107
A study of three successive influenza seasons (2006–2009) showed that hospitalisation rates for RSV and hMPV in adults aged ≥50 years were substantial, and similar to those associated with influenza (Figure 3)109. In a meta-analysis from 2015, the incidence of RSV-associated acute respiratory infection in older adults in industrialised countries was an estimated 6.7 cases/1,000 persons per year (95% confidence interval [CI]: 1.4–31.5).110 This represents an estimated 1.5 million cases, 214,000 of which involved hospital admission.110 A retrospective cohort study from 2019 of hospitalised older adults (≥60 years) showed that RSV infection was associated with greater odds compared with influenza virus infection for hospital length of stay ≥7 days (p<0.001), pneumonia (p<0.001), intensive care unit admission (p=0.023), exacerbation of COPD (p=0.001), and mortality within 1 year of admission (p=0.019).111 Osterhaus stated: “RSV infection causes serious and life-threatening respiratory illness, and is comparable to, or more serious than, influenza. This is a new and important message for healthcare professionals.”
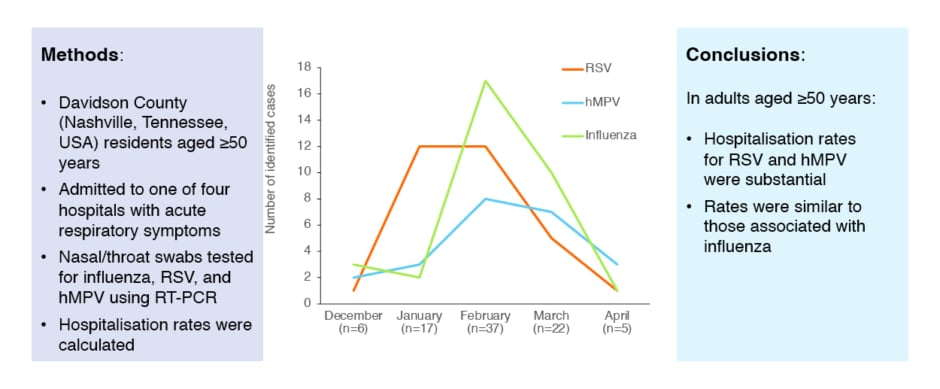
Figure 3: Seasonal variation in hospitalisations for respiratory viral illness across three successive influenza seasons (2006–2009).
hMPV: human metapneumovirus; RSV: respiratory syncytial virus; RT-PCR: real-time reverse-transcriptase PCR.
The same results were first published in Widmer et al. 2012;109 the graph has been independently created by GSK from the original data.
A meta-analysis in 2020 showed the substantial burden of RSV in the USA, with an estimated 177,000 hospitalisations and 14,000 deaths annually in adults aged >65 years infected with this virus.112 In contrast, there were 52,527 hospitalisations annually in children aged <5 years.112 In a global study during the 2017–2019 epidemic seasons (published in 2021), influenza-related hospitalisations in adults were more common than RSV- or hMPV-related hospitalisations, but patients with RSV or hMPV infections had a greater frequency of underlying risk factors, and medical resource utilisation.113
A 2022 study showed the seasonal distribution of cases of RSV infection tends to be more dispersed than that of influenza virus infection,114 whereas patterns of comorbidities and outcomes for the two infections are similar.114 A meta-analysis in 2022 of 14 studies describing the incidence of medically-attended RSV among adults in the USA showed that the true burden of RSV infection has been underestimated, and is significant in older adults and individuals with chronic medical conditions.115 Most recently, in a meta-analysis in 2023 of 16 studies, RSV disease burden in adults aged ≥60 years in high‐income countries was assessed in terms of the attack rate, defined as the number of new cases of RSV-associated acute respiratory infection during a specified time interval divided by the size of the population at risk, and the in‐hospital case fatality rate.116 The attack rate was 1.615% (95% CI: 0.840–3.081) and the in‐hospital case fatality rate was 7.133% (95% CI: 5.404–9.361), which Osterhaus considered high, and an indication that RSV infection is potentially serious in older adults.
Conclusion
Birgit Weinberger, Tino Schwarz, and Albert Osterhaus
Weinberger summarised that the burden of infectious disease is considerable in older individuals and other risk groups, and is broader than just acute disease, including increased risk of cardiovascular events after infections, exacerbations of comorbidities, frailty, and loss of independence.14-16,20-23 Vaccination against many infectious diseases is available for adults.117 Optimal vaccine uptake is essential for optimal protection; however, vaccination recommendations and coverage vary greatly between European countries.26,28,31 There is a major need to reverse the trend of decreased vaccine coverage in adults in Europe and improve vaccine coverage, as well as to harmonise guideline recommendations.
Schwarz synopsised that the incidence of HZ increases with age from 50 years onwards,5,35 and several underlying diseases are associated with an increased risk of HZ from 18 years.3 The clinical presentation of HZ in immunocompromised populations can be atypical and more severe, with a higher risk of HZ and HZ complications compared with the general population.75,77,82 Clinical trials with RZV have demonstrated a high vaccine efficacy in patients with various underlying diseases,91 and in patients who are immunocompromised.87,92 There is a need for education of primary care physicians and specialists on the potential impact of HZ on long-term health, particularly in vulnerable populations.
Osterhaus concluded that virtually all children have been infected with RSV at least once by the age of 5 years,100 and RSV re-infections occur throughout life.100 Although clinicians still mostly regard RSV infection as a disease of babies, infants and children,107,108 RSV is a major respiratory pathogen for older adults (>65 years).87,91 RSV infection is comparable to influenza virus and hMPV infections in this population.100,109 Awareness and diagnosis of RSV infection need to be improved, and the therapeutic landscape is changing. Osterhaus proposed that point-of-care diagnostic capabilities are needed for RSV, and vaccines and antiviral therapies are awaited with interest.
Key Questions from the Question- and-Answer Session
Birgit Weinberger, Tino Schwarz, and Albert Osterhaus
What Are the Recommendations for the Timing of Recombinant Zoster Vaccine Doses in Patients Who Are About to Start Immunobiological Treatments?
Schwarz stated that the interval between the two vaccine doses can be shortened from 2 months to 1 month for these patients.88 If clinically appropriate, it may be preferable for both vaccine doses to be administered before starting immunobiological treatment.
Is There Any Signal that Recombinant Zoster Vaccine Can Prevent Other A-herpes Virus Infections or Reduce Relapses?
Schwarz said that there is no signal that anything other than VZV is tackled. The glycoprotein is specific for VZV.
Is It True that Our Chronological Age Does Not Correlate with Our Biological Age?
Weinberger said that this is true. As with all other organ systems, the immune system does not age the same way for everybody. Also, the individual components of the immune system do not age at the same rate. There is a spectrum of functionality within the immune system that is very individual, and effectors other than age, such as cytomegalovirus, are also important.
In the Era of Personalised Medicine, Is It Possible to Identify a Time Point When These Interventions Should Be Made?
Weinberger stated that it is important to look at comorbidities. The age at which there is a shift to increased risk is clear in HZ,5 but not in other infectious diseases. The age-associated recommendations for interventions make a lot of sense, and make life easier. Personalisation of treatment is worthwhile when there is a lot of benefit; however, the easier the recommendation, the better the compliance.
What About a Vaccine Against Respiratory Syncytial Virus for Children?
Osterhaus said that strategies for RSV in children include monoclonal antibodies.105 Vaccine development for children is ongoing, but clinical development takes longer. Another strategy is maternal vaccination, provided antibody levels are high enough.
Respiratory Syncytial Virus Is an Underdiagnosed Infectious Disease. How Do We Bridge the Data Gap?
Osterhaus said that looking at all the meta-analyses, the data are there. The risk criteria are being fine-tuned. What we need now is a vaccine.
How Will Payers Fund Vaccines for Influenza, Herpes Zoster, and Respiratory Syncytial Virus Infection?
The experts agreed that this depends on the geographical location and the political goals surrounding the health of elderly populations. Healthy ageing is better than unhealthy ageing, because that is more costly. Major considerations include vaccine hesitancy, reimbursement, accessibility, and social media influence regarding burden of disease.
©️ 2023 GSK group of companies or its licensor.
GlaxoSmithKline Biologicals SA, Rixensart, Belgium
NX-GBL-GVU-JRNA-230001 | June 2023






