Meeting Summary
Clinical trials show that non-vitamin K antagonist oral anticoagulants (NOACs) have good efficacy-safety profiles relative to warfarin across a broad spectrum of patients with non-valvular atrial fibrillation (NVAF). These findings are currently being confirmed for rivaroxaban through real-world evidence, with results from these studies consistent with results from Phase III randomised controlled trials (RCTs). Of all the NOACs, rivaroxaban currently has the most extensive real-world experience across different data sources (prospective and retrospective registries, database analyses, and prospective studies). Anticoagulant-related bleeding is still a concern amongst clinicians, however awareness of patient characteristics and other factors that can increase bleeding risk can assist in the proactive and effective management of bleeding episodes. Particularly, in atrial fibrillation (AF) patients with renal impairment who have an incrementally higher risk of bleeding and stroke, administration of NOACs versus vitamin K antagonists (VKAs) is beneficial. When dosed appropriately, NOACs such as rivaroxaban are effective in patients with renal impairment and offer an alternative to warfarin, with increased efficacy and decreased risk of critical bleeding events.
From Large-Scale Study to Larger-Scale Practice: How do Real-World Findings for Non-Vitamin K Antagonist Oral Anticoagulants Stack Up?
Professor Manesh R. Patel and Professor Eric D. Peterson
Results from RCTs are not always mirrored by evidence from real-world studies, which are inclusive of a more varied clinical setting and have a diverse patient population(s) more reflective of routine clinical practice.1 Often, results reported in RCTs can depend upon the selection of the patient population studied; for example, in four trials investigating the use of NOACs, ARISTOTLE,2 RE-LY,3 ENGAGE AF,4 and ROCKET AF,5 estimation of stroke risk in AF using the CHADS2 (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke, or transient ischaemic attack) score system, found that the patient populations in these studies had varying CHADS2 scores. In ARISTOTLE,2 RE-LY,3 and ENGAGE AF,4 30%, 32%, and 53% of AF patients had a CHADS2 score of 3–6, respectively; in contrast the ROCKET AF trial,5 in which 87% of patients had a CHADS2 score of 3–6, indicated that AF patients had a higher risk of stroke than patients in the other RCTs. The differences in patient characteristics across these trials, particularly in risk factor profiles, can make comparing trial outcomes for NOACs challenging. Data from two large registries, GARFIELD-AF6 and ORBIT-AF,7,8 which enrolled patients across the globe, demonstrated important differences between demographics of the AF population treated, particularly with regard to CHADS2 scores.
A meta-analysis of Phase III trials from all four licenced NOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) has shown that NOACs as a group deliver a greater benefit than risk, and are associated with significant reductions in haemorrhagic stroke (with a strong trend towards lower rates of ischaemic stroke), all-cause mortality (with a trend towards lower rates of myocardial infarction), and intracranial haemorrhage (ICH) when compared with warfarin therapy, although they also demonstrate an increased risk of gastrointestinal (GI) bleeding (Figure 1).9 The meta-analyses reported the relative efficacy and safety of these NOACs as consistent across a wide range of AF patients,9 but it is important to note that the usual limitations of meta-analyses mean that the efficacy and safety of the individual NOACs cannot be reliably compared.
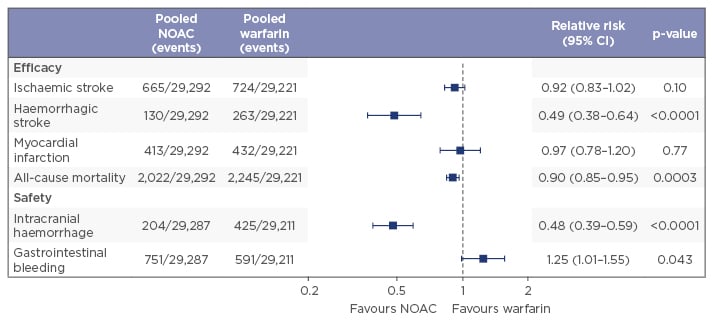
Figure 1: Anticoagulants deliver greater benefit than risk, NOACs more than VKAs.9
NOACs: non-vitamin K antagonist oral anticoagulants; CI: confidence interval; VKAs: vitamin K antagonists.
In the real-world, prospective, observational XANTUS study, patients treated with rivaroxaban for stroke prevention in AF had low rates of both stroke (events per 100 patient-years: 0.7; 95% confidence interval [CI]: 0.5–0.9) and major bleeding (events per 100 patient-years: 2.1; 95% CI: 1.8–2.5),10 reassuringly reflecting the safety profile observed in clinical trials as well as demonstrating effectiveness. The rate of stroke and major bleeding with rivaroxaban was also low across the Dresden NOAC and US Department of Defence real-world studies, consistent with results from ROCKET AF, although it must be noted that the mean CHADS2 score was also lower in these real-world studies which may have contributed to the lower event rates.5,10-12 Another recently published retrospective database analysis, the REVISIT-US study, compared the effectiveness and safety of rivaroxaban and apixaban relative to with warfarin in NVAF.13 While both rivaroxaban and apixaban significantly reduced ICH versus VKAs (p<0.05), only rivaroxaban was associated with a non-significant decrease in ischaemic stroke versus warfarin, whereas for apixaban versus warfarin there was a non-significant increase in ischaemic stroke observed.13
Large-scale registry data show that oral anticoagulants in general are underused in those patients who stand to benefit the most from them. The underuse of anticoagulant therapy has been reported in the aforementioned GARFIELD-AF registry, where higher-risk patients (CHADS2 score ≥2) were generally under-treated with 38% not receiving anticoagulant therapy, placing them at a higher risk of stroke.6 Conversely, the same study found that 42.5% of low-risk patients (CHADS2 score 0) were generally over-treated.6 Although observational study of patients in community clinical practice can provide important data, limitations of such a study include enrolment or sampling biases and reporting bias, which may not give a true account of over versus under-dosing. An analysis from the ORBIT AF registry describes the proportion of patients on warfarin who had stable international normalised ratio (INR) values over an 18-month period.14 The study found that only 26% of patients had stable INR values (2.0–3.0) over a 6-month baseline period and of these only 34% had stable INRs the following year; an indication that predicting INR stability on warfarin therapy is a difficult task for clinicians.
The appropriate dosing of the approved NOACs is an important topic and the criteria for dose adjustment of NOACs varies across RCTs. For example, in ROCKET AF, dose adjustment from 20 mg once daily to 15 mg once daily was made in 20.7% of patients, on the basis of renal insufficiency as measured by creatinine clearance (CrCl).15 In ENGAGE-AF9,16 and ARISTOTLE,2 dose adjustments were made throughout the trial (ENGAGE-AF) or at random (ARISTOTLE) on one of a number of criteria (ENGAGE: dose adjustments at random or throughout for ≥1 of the following: CrCl 30–49 mL/min, weight ≤60 kg, strong P-glycoprotein [P-gp] inhibitors; ARISTOTLE: dose adjustments at random if ≥2 of the following: age ≥80 years, weight ≤60 kg, creatinine ≥133 mmol/L) in 25.4% and 4.7% of patients, respectively, whilst in RE-LY3 there was no dose adjustment and patients were randomised to be treated with one of two doses, in contrast to the other RCTs mentioned (49.7% of patients were receiving the dabigatran 110 mg twice daily dose whilst 50.3 received the 150 mg twice daily dose).
Prescribing patterns across the globe show that, in practice, prescriptions for apixaban at the lower 2.5 mg dose are disproportionately high. Similar but less-marked patterns are also seen with dabigatran and rivaroxaban, although dose reduction with rivaroxaban and dabigatran offers the flexibility of a 25% or 27% dose reduction, respectively, whereas apixaban only offers the option of a 50% dose reduction.2,3,15,17 Generally, preferences of physicians regarding doses of oral anticoagulant therapy are often based on the safety of the therapy, and physicians are generally more concerned about bleeding risk than stroke prevention. Dosing regimens should also be considered by clinicians, particularly with regards to adherence (the extent to which a patient acts in accordance with the prescribed length of treatment, frequency, and dose of a dosing regimen). In a Canadian analysis, 6% and 14% of patients who received rivaroxaban and warfarin, respectively, reported taking their oral anticoagulant twice daily instead of once daily, but importantly, 27% and 30% of patients who received dabigatran and apixaban, respectively, took their oral anticoagulant once daily instead of their recommended twice daily dosing regimens. There were significantly more missed doses with the twice daily dosing regimen compared with once daily medications.18 Comparisons of discontinuation rates of rivaroxaban versus warfarin in real-world studies have shown a 34–37% lower risk of discontinuation with rivaroxaban versus warfarin.19,20
Overall, results from RCTs and real-world studies of rivaroxaban provide complimentary and consistent evidence that when dosed appropriately, NOACs such as rivaroxaban are effective and safe in patients in comparison to warfarin.
Day-to-Day Management of Patients with Atrial Fibrillation on Non-Vitamin K Antagonist Oral Anticoagulant Therapy: Practical Perspectives
Professor Peter Verhamme
The development of NOACs has changed the therapeutic landscape of anticoagulant therapy and provided patients with a therapeutic option that has a reduced risk of critical bleeding events versus VKAs. The ROCKET AF trial has shown that rivaroxaban reduces incidence of critical bleeding events versus VKAs in patients with AF; critical organ bleeding is reduced by 31% (p=0.007), ICH by 33% (p=0.02), and fatal bleeding by 50% (p=0.003).5,21 In addition to a lower risk of critical bleeding events, NOACs also have a different bleeding pattern in comparison to VKAs and demonstrate a lower relative risk of ICH and other major bleeding events. This is likely due to the difference in mechanism of action of NOACs, which act to directly inhibit factor Xa (rivaroxaban, apixaban, edoxaban) or thrombin (dabigatran), whereas warfarin reduces the functional levels of the coagulation factors II, VII, IX, and X.22 Given the reduction in all non-GI bleeding, it is surprising that the risk of GI bleeding is increased with NOACs as a group versus warfarin.9 One explanation may be the accumulation of active drug in the GI tract where NOACs have the potential to cause bleeding through both their systemic and local effects on the GI mucosa, in contrast to VKAs which can only cause GI bleeding through a systemic anticoagulant effect.22
When interpreting bleeding outcome findings for NOACs versus warfarin, it is also important to consider differences in patient characteristics across NOAC trials. High-risk patients (CHADS2 score ≥3) had a higher risk of major bleeding in the ROCKET AF trial (2.0% major GI bleeding event rate/year; mean CHADS2 score 3.5).5 In the real-world study XANTUS, the major GI bleeding event rate/year was lower (0.9%; mean CHADS2 score 2) than that seen in ROCKET AF, but is likely due to the difference in patient populations investigated in both studies.10,23 A meta-analysis of observational cohort studies (N=8) examined the link between NOACs and real-world GI bleeding events.24 The analysis included 10,713 patients treated with rivaroxaban and reported no significant increase in GI bleeding risk for rivaroxaban versus warfarin,24 probably attributed to the fact that patients in the real-world setting generally have a lower risk profile than the patients recruited to ROCKET AF; this is reflected in lower major bleeding rates. Proactive measures can be taken to lower bleeding risk in patients on oral anticoagulants. Risk factors for bleeding include older age, male sex, high diastolic blood pressure, the use of platelet inhibitors, a history of GI bleeding, and anaemia.25 Clinical guidelines call for clinicians to consider these risk factors when administering anticoagulant therapy with a more integrated clinical approach.26 Another concern among clinicians and patients is traumatic ICH, particularly among older patients with AF who may be more prone to falls.27 In a retrospective cohort study of 31,951 USA veterans (≥75 years) with AF, newly referred to anticoagulation clinics for VKA therapy (2002–2012), the incidence rate of hospitalisation for traumatic ICH was 0.48% per year, and of any ICH, 1.46% per year.27 These results indicate that rates of traumatic ICH with VKAs may be higher than previously thought.
Initial management of serious bleeding events include identifying and controlling the source of the bleed and providing supportive care to stabilise the patient, using volume replacement and transfusion. Assessing the type and amount of drug as well as the time of administration, measurement of haemoglobin, and haemostasis (partial thrombin or activated partial thromboplastin time)28,29 are also important considerations in bleeding management. In particular, supporting haemostasis using either procoagulants (prothrombin complex concentrates [PCCs]) or antifibrinolytics, or using reversal agents such as idarucizumab (not available in some countries) and andexanet (which has not yet received regulatory approval) may help with serious bleeding events in exceptional cases. In a randomised, double-blind, placebo-controlled study in which 12 healthy male volunteers received rivaroxaban 20 mg twice daily, followed by either a single bolus of 50 IU/kg PCC (Cofact) or a similar volume of saline, prolongation of partial thrombin and endogenous thrombin potential were completely reversed by PCC.30 The European Heart Rhythm Association (EHRA) dosing recommendation for factor concentrates recommend PCC (50 U/kg), activated PCC (50 U/kg/day; maximum 200 U/kg/day), or recombinant factor VIIa (90 mg/kg) in patients with NVAF.28 In fact, standard clinical measures are often sufficient to manage major bleeding in the majority of cases; in the Dresden NOAC registry, <10% of patients with major bleeds received PCCs and no patients received recombinant factor VII.31 More recently, interim results with reversal agents idarucizumab, which completely reverses the anticoagulant effect of dabigatran,32 and andexanet, which reverses the anticoagulant effect of apixaban and rivaroxaban,33 potentially offer additional alternatives in specific individual situations.
Less critical bleeding with NOACs and a more comprehensive understanding of what drives bleeding risk, plus a more informed and proactive approach to management of bleeding means that in the future bleeding risk is likely to be lower with anticoagulant therapy, offering better therapeutic options for clinicians and patients alike.
The Management of Patients with Atrial Fibrillation and Renal Impairment: Practical Perspectives
Professor Jafna L. Cox
A frequently asked question is how to treat renally-impaired patients with anticoagulant therapy, particularly those that are elderly. Data have shown that chronic kidney disease (CKD) is associated with an increased risk of stroke, systemic thromboembolism, and bleeding. Renally-impaired populations differed between NOAC Phase III trials,15,34,35 as did the CHADS2 score distribution across studies for patients with moderate renal impairment (CrCl 30–49 mL/min) (Figure 2). Of the patients with renal impairment, 45% had a CHADS2 score of 3–6 in ARISTOTLE34 and RE-LY,35 whereas this number was much greater in ROCKET AF (91%), indicating a population at substantially higher risk of stroke and bleeding.15
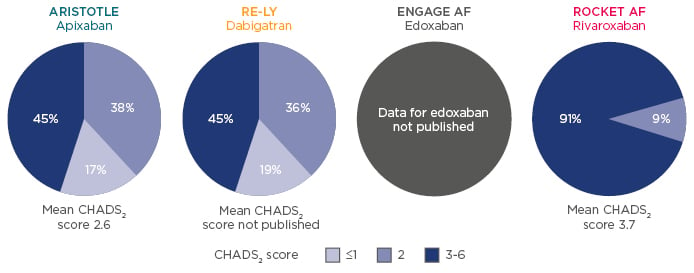
Figure 2: Renally-impaired populations differ between Phase III studies.15,34,35
A subgroup analysis of the meta-analysis of Phase III trials on NOACs and stroke prevention in NVAF by Ruff et al.9 found that 19% of patients with renal impairment had a CrCl of <50 mL/min. The assessment found that treatment with NOACs of NVAF patients with renal impairment reduced the relative risk of stroke, systemic embolism, and major bleeding versus warfarin.9
Rivaroxaban is currently the only NOAC that has a prospectively tested, specific renal dose for patients with renal impairment that has been clearly assessed in a Phase III study.36 ROCKET AF has demonstrated that rivaroxaban has a consistent efficacy and safety profile in NVAF patients with moderate renal impairment (CrCl 30–49 mL/min), and is associated with a significant 61% reduction in fatal bleeding events in this patient group. ICH and critical organ bleeding were also numerically lower versus warfarin therapy.15 Additionally, a ROCKET AF sub-study aimed to determine whether the primary efficacy (stroke or systemic embolism) and safety (major bleeding and non-major clinically relevant bleeding) endpoints in the parent trial differed among participants with worsening renal function (defined as an absolute increase in serum creatinine) taking rivaroxaban versus warfarin. It found that among such patients with worsening renal function, rivaroxaban was associated with lower rates of stroke and systemic embolism compared with warfarin; importantly, this benefit was seen without any corresponding increase in the composite bleeding endpoint.37
Studying the real-world use of NOACs provides important insights into the effects of oral anticoagulant therapy in AF and CKD. A study of the Danish National Registry (1997–2008, including 132,372 patients with 3,587 patients with non-end-stage CKD [2.7%]) found that both AF and CKD significantly increased the risk of stroke (p<0.001) and bleeding (p<0.001) among patients with CKD, versus those patients without CKD.38 A Swedish AF Cohort study has found similar results. This study comprised 307,351 patients with AF, of whom 13,435 had a previous diagnosis of renal failure (28% of whom were on warfarin therapy at baseline). Other baseline characteristics across patients with renal failure and with no renal failure were similar, however when the groups were stratified both by bleeding and stroke risk, the risk in each case was higher in the renal failure group. Interestingly and as previously discussed, the patients most at risk of stroke were under-dosed (warfarin at baseline) by 28%,39 presumably out of fear of bleeding risk. However, in this study patients with both AF and renal failure were those found to benefit most from having the same anticoagulation treatment as is recommended for other patients with AF. As such, rather than be undertreated, they should be at least as aggressively treated as AF patients with normal renal function, if not more so. Adding additional points for renal failure to the CHADS2 and CHA2DS2-VASc (with the additional ‘stroke risk’ modifiers of vascular disease, age 65–74 years, and a particular sex category) scores did not improve their predictive value.39
XARENO, an ongoing real-world study of rivaroxaban in patients with renal impairment, aims to assess CKD progression and the safety of rivaroxaban versus VKAs in NVAF patients with estimated glomerular filtration rates of 15–49 mL/min/1.73 m2 in routine clinical practice. Patient selection and choice of type, dose, and duration of drug used will be at the discretion of the attending physician, and patients will either receive rivaroxaban, warfarin, or no oral anticoagulant for ≥3 months. Investigators will collect data at the initial visit, at 3 months, and then quarterly. The registry will collect clinical data relating to approximately 2,500 patients with CKD over an estimated mean follow-up of 18 months for the whole study cohort, and thereby offer important insights into the use of factor Xa inhibitors in renally-impaired patients with NVAF.40
Hub Session: Impact of Real-World Evidence on Patient Management: Addressing Open Questions
The aim of the Hub session was to provide an in-depth debate around the implications of clinical trial and real-world evidence.
Professor Manesh R. Patel
The GARFIELD-AF observational study has shown that patients with AF are not treated according to current guidelines, with under-treatment with anticoagulants in 38% of patients with CHADS2 scores ≥2 (as previously mentioned).6 There is often discrepancy between RCTs and real-world evidence in the treatment of clinically-challenged patients, such as those with renal impairment. Would the audience agree or disagree that real-world evidence is more important than Phase III clinical trial data for ascertaining expected drug effectiveness/safety?
Audience
There was a 50/50 split between agree and disagree.
Professor Jafna L. Cox
Real-world evidence, which is based on data from routine clinical practice, can provide valuable insights and knowledge for physicians to support patient care. Although RCTs are the gold standard of evidence-based medicine and feature highly controlled settings with predefined patient types and limited follow-up times, data from routine clinical practice generally involve varied medical settings with diverse patient populations.1 Both types of data are important and can be viewed as complementary, as they address different questions.
Professor Christoph Bode
When looking at the XANTUS registry data, results from this study confirm those from clinical trials: rivaroxaban is highly effective and has a good safety profile.
Professor Peter Verhamme
In the case of bleeding control, management using standard clinical measures has been reported to be sufficient in the Dresden NOAC registry.31
Professor Manesh R. Patel
Using NOACs ‘as they were tested’ in patients with AF is critical to providing adequate stroke prophylaxis.
Professor Jafna L. Cox
Most clinical trials test specific dosages of drug, often without pre-specified dose titration, giving us limited insight into the effectiveness and safety of untested prescription amounts. However, many clinicians tend to take a conservative approach, often opting for lower doses of anticoagulant because of fear of bleeding.
Professor Manesh R. Patel
When prescribing NOACs for patients with AF, the simplicity of the dosing regime is an important consideration.
Professor Jafna L. Cox
It is important to have discussions with your patients about the dosing regimen they would prefer. Often complicated regimens with 2 or 3-times daily dosing is not the patient’s preferred choice and can lead to reduced adherence.








