Summary
Anaemia is a common and serious complication of chronic kidney disease (CKD) that can greatly impact the daily lives of patients. However, poor awareness around anaemia of CKD (aCKD), from both physicians and patients, may impede its identification and treatment. During interviews conducted by EMJ in April 2023, leading nephrologist Christoph Wanner, University Hospital of Würzburg, Germany, and two patients/patient advocates, Daniel Gallego and Jemma Reast, gave their informed opinions on this topic. From their different viewpoints, they described how greater understanding of symptoms and treatment options could empower patients to make better choices for their own care. At the same time, they considered how greater physician awareness of aCKD, and the human impact beyond haemoglobin levels could influence diagnosis and treatment priorities. Aligning these two perspectives, they also discussed the powerful benefits of improved communication and shared decision-making between patient and physician, and its potential for relieving the burden of aCKD.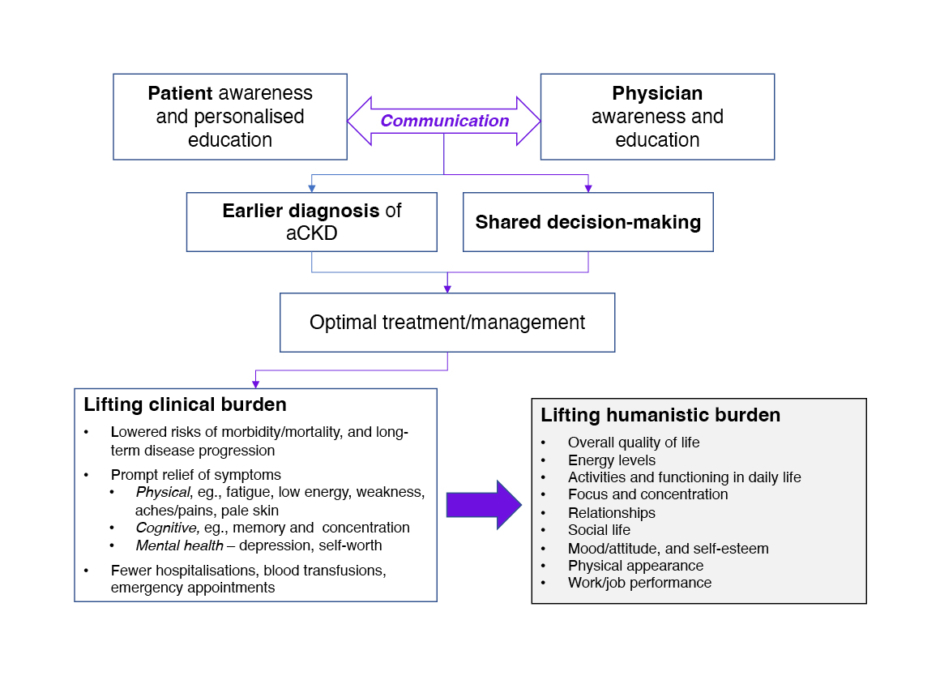
Figure 1: Benefits of aligning patient and physician awareness of anaemia of chronic kidney disease.
aCKD: anaemia in chronic kidney disease.
![]()
Interviewee Biographies
Christoph WannerProfessor of Medicine and Chief of the Division of Nephrology and Hypertension, University Hospital of Würzburg, Germany; President of European Renal Association (ERA)
Christoph Wanner’s research in the field of chronic kidney disease of diabetic and non-diabetic origin has led to the overseeing of large outcome trials to improve the life of patients with kidney disease and preserve kidney function. Wanner has been Principal Investigator of the 4D study, and steering committee member of the SHARP, EMPA-REG Outcome, EMPA-KIDNEY, and ASCEND trials. Wanner has published more than 900 PubMed-referenced scientific articles. In 2012, they received a doctor honoris causa from the Charles University of Prague, Czechia, and the highest awards from the European Renal Association (ERA) and the German Society of Nephrology.
Daniel GallegoPatient with CKD, Spain; President of the European Kidney Patients’ Federation (EKPF)
Daniel Gallego was diagnosed with CKD in 1993, aged 19 years, following a visit to the emergency department with a suspected sprained ankle. Gallego began haemodialysis in 1995, and received a kidney transplant in 1998, which subsequently failed. They remain on haemodialysis, and have experienced the symptoms of anaemia throughout their life with CKD. Alongside their considerable experience as a patient, Gallego is President of the EKPF, and was formerly President of the Spanish Kidney Patient Federation (Association for the Fight Against Kidney Diseases [ALCER]), working to improve lives and offer hope for people living with CKD.
Jemma ReastPatient with CKD, UK; advocate for patient-led healthcare, London Kidney Network (LKN), UK
Jemma Reast was diagnosed with CKD aged 2 years, following an acute kidney infection (E. coli) in 1997. At age 15 years, Reast’s condition went into rapid decline, and they underwent a kidney transplant from their father. Shortly afterwards, they developed haemolytic-uremic syndrome, which caused significant damage and scarring to the donor kidney. Consequently, and with signs of long-term organ rejection, Reast required a second kidney transplant at age 24 years. They remained on peritoneal dialysis for 2 years during the COVID-19 pandemic, during which anaemia was a significant problem, before receiving a transplant from their sister. Reast currently works in healthcare research, and is also an advocate for patient-led healthcare with the London Kidney Network (LKN), UK.
![]()
BACKGROUND TO ANAEMIA OF CHRONIC KIDNEY DISEASE
CKD is a complex condition that involves many different body systems and symptoms, alongside the risk of serious complications such as anaemia.1 Exacerbating the heavy burden of CKD, anaemia can negatively impact morbidity, mortality, and healthcare resources, as well as the daily functioning and emotional health of patients and their families.2-5 However, differences between patients’ and physicians’ perceptions around anaemia and its treatment in CKD (aCKD)6 could affect patient care. To provide insight on this topic, the views of a clinical expert in CKD, Christoph Wanner, and two patients/patient advocates with CKD, Daniel Gallego and Jemma Reast, were sought.
CKD is characterised by a decline in kidney function (measured as estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2), or by moderately increased or severely increased albuminuria (urinary albumin–creatinine ratio: ≥3 mg/mmol) with well-preserved kidney function.7 Studies have demonstrated that anaemia of CKD, defined as haemoglobin <13.0/12.0 g/dL for adult males and females who are not pregnant,8 is associated with an increased risk of CKD progression, major cardiovascular events, and all-cause mortality.3,5 Wanner explained: “Dysfunction of the heart is the most prominent comorbidity in CKD, and observational studies show that anaemia affects left ventricular function.9-12 Patients with anaemia of CKD can have a shorter lifespan, due to the considerable comorbidity of cardiovascular disease, and anaemia is a very common complication of CKD.” Due to this potentially severe clinical impact, Wanner recommends screening for anaemia from the mild/moderate stages of CKD (Stage 3; eGFR: 30–60 mL/min/1.73 m2), although they noted it becomes more common as the disease progresses, estimating that 8 out of 10 people who enter dialysis have anaemia.
THE IMPACT OF ANAEMIA FOR PATIENTS WITH CHRONIC KIDNEY DISEASE
Wanner said that tiredness and fatigue, and lack of energy are the two main symptoms reported by patients with aCKD. However, based on their own patient experiences, Gallego and Reast emphasised that the impact of anaemia goes far beyond a discrete list of symptoms. “Anaemia is so multifaceted, and it can affect not just you but your whole circle,” said Reast. “For physical symptoms like extreme tiredness, the impact is not being able to work, or have the energy to do what you want to. I am quite an active person, but when I was at my most anaemic I was struggling with heavy legs and back pains, and couldn’t even get up a short flight of stairs. Then there’s the cognitive side. People talk about ‘brain fog’ in CKD, but don’t necessarily talk about how it can be connected to, and exacerbated by, anaemia. I would forget words and wouldn’t really want to converse, so I just shut myself away. I was lucky that my employer was really understanding, and I was able to get work-related health assessments, but I know a lot of people are not so lucky.” Gallego added: “When I was diagnosed, I felt devastated because I didn’t want to live with this condition all my life. I felt really, really tired, but didn’t know about the importance of haemoglobin or iron. Anaemia is a real disability because you feel powerless, you lack energy and focus, and you are not able to keep up with what you did before, so you feel frustrated and disappointed. Importantly, it also affects physical appearance and self-esteem, which really matter to patients.”
Reast was also frank about having struggled with mental health in CKD, describing how fatigue had affected their self-worth: “If you don’t feel you can sustain relationships or provide value to other people because you’ve got no energy, it can be really demoralising because you feel completely worthless. It changes your whole personality.” Gallego concurred: “Sometimes the symptoms of anaemia are very similar to those of depression: tiredness, lack of energy, feeling disappointed and frustrated.” In a particularly powerful example, Reast described a challenging period pre-transplant when it was not just their own mental health that was affected: “I could barely walk. It was really distressing for me, and also for my boyfriend who was looking after me. He’d previously known me as this really healthy, active person, and due to what he saw me go through, he’s had to go to therapy. I think a lot of that was down to me being so severely anaemic.”
DIAGNOSIS AND MONITORING OF ANAEMIA
Despite the marked impact of anaemia symptoms on patients’ daily lives, the interviewees spoke of how diagnosis and subsequent monitoring of anaemia remains centred on clinical (haemoglobin) testing, highlighting a sharp contrast in patient versus physician perceptions. Wanner observed: “The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines for anaemia of CKD were issued over 10 years ago,8 and over the many years of anaemia treatment [since the arrival of erythropoietin in the 1980s], we have arrived at this broad, easy-going practice in our nephrology clinics, overfamiliarity perhaps, paying less attention, and just measuring haemoglobin because it is easy and cheap. Listening takes time, so there is a tendency not to take account of a patient’s signs and symptoms when haemoglobin levels are still within [the accepted] range.” Reast’s and Gallego’s experiences as patients also reflected this situation, with minimal follow-up of their anaemia, aside from blood tests, taken at 3–6 monthly check-ups.
However, all three interviewees felt that change was needed, and that patient-reported signs and symptoms should be considered when assessing anaemia across the course of CKD. “What patients feel should be important, and at the moment doctors have forgotten this,” remarked Wanner. Gallego agreed: “There needs to be a focus on the clinical impact of CKD, of course, but once survival is ensured then we need to try to tackle the humanistic burden of this chronic disease. What really matters to the kidney patients is health-related quality of life (QoL), daily life activities, and social and life participation.”
This is consistent with the findings of a survey from the International Consortium for Health Outcomes Measurement (ICHOM), in which patients identified fatigue as amongst the most important outcome measures for assessing CKD, alongside survival, daily function/activities, and overall QoL.13 It was suggested that better awareness amongst physicians of how anaemia presents in patients’ daily lives, and the impact this has, could not only improve management, but also help with earlier diagnosis. Wanner explained: “At the moment, nephrologists’ time is greatly focused on addressing the progression of kidney disease because we have fantastic medications, and so anaemia is pushed to the second line. However, if anaemia isn’t diagnosed promptly, it can have an impact on the patient’s long-term condition. The pathology clearly advances.” In short, it is felt that anaemia needs to be a greater priority in the minds of physicians.
PATIENT AWARENESS
In the period around diagnosis, any discussion of anaemia with the patient was said to be largely solution or treatment-based, rather than involving any background or education to help with understanding the condition. In particular, Reast spoke of a disconnect between the symptoms they had experienced, and any knowledge that these were due to anaemia rather than CKD itself: “There was never an obvious link. I was told that my haemoglobin was a bit low, which meant I was anaemic, and I was going to be given treatments to help me have more energy, and it was only brought up when it was a problem. The first time I realised it was related to CKD was when I was waiting for my first transplant and I started receiving [erythropoietin] injections, but it was only a couple of years ago that I really understood that I had anaemia and I also had CKD, and that there was a link between the two. Previously, I had put my anaemia symptoms down to general CKD, and I think many people do that. There’s a lot of resignation because CKD is known to be difficult, and the tendency is just to get on with it.” Gallego summarised the situation: “You think it’s all because of the CKD, and I think we need to change that.”
These accounts suggest that patient education around aCKD is currently inadequate. Gallego commented: “Nobody gave me leaflets or information about available resources regarding anaemia, and it is really difficult for CKD patients to find patient-specific materials related to anaemia. Scientific papers and guidelines are too technical and detailed for patient communications.” Reast described their knowledge of anaemia as being mostly self-taught, although they acknowledged that receiving all the information at the point of diagnosis would have been overwhelming, and likely too complex to fully comprehend. “I generally learned bits here and there along the way, and through talking to other patients,” they said. “Whilst I was on dialysis there was a bit more conversation around anaemia, but I wasn’t given anything or referred to any resources on anaemia specifically, so I did my own research because I wanted to know more.”
Alarmingly, Reast’s initial lack of understanding around anaemia affected their treatment compliance, and led to a crisis point while they were waiting for a transplant: “I didn’t do my [erythropoietin] injections the way I should have done. Then, I refused to get the blood transfusions I needed, until it reached the point where they wouldn’t let me leave the hospital without giving me multiple units of blood. I was scared. I was on the transplant list and didn’t want antibodies to impact my likelihood of being a viable recipient, and that was a barrier in my way of thinking. I was lucky that those blood transfusions didn’t cause any antibodies, but I am shocked and sad that I let it go on that long, and also that other people didn’t think to intervene sooner or more strongly, or give me more information. Once I understood more, it made my ability to be fully adherent so much easier. I still hated doing the injections, but because I’d seen the benefit, I knew that I had to keep doing them.”
Patient Versus Physician Priorities
The gap in patient awareness and education around aCKD may reflect a difference in priorities between patient and physician. Gallego commented: “Clinical impact is really important for the professionals, and I understand why. I think when you report symptoms such as tiredness or lack of energy, the nephrologist does not think about anaemia, but about CKD overall, and the reaction is to say that it’s just part of the disease.” Reast added that physician priorities may also be influenced by the most urgent or controllable needs, especially within a time-constrained consultation: “To an extent, I don’t think physician and patient priorities are very different, but it’s that there’s so much going in CKD. Renal specialists are time-poor, so therefore focus very much on what they can control.” Reast continued to explain that patient awareness can also influence physician priorities; for instance, the patient may find it easier to describe or recognise issues such as itching, which may be related to high phosphate levels, or dietary or fluid restrictions, while the signs of anaemia may be harder to identify. Thus, communication and the balance between physician awareness (asking the right questions) and patient awareness (raising the right concerns) are key.
TREATMENT OF ANAEMIA OF CHRONIC KIDNEY DISEASE
The 2012 KDIGO Guidelines for anaemia in patients with CKD recommend iron (oral or intravenous), erythropoietin-stimulating agents, and, in urgent and acute cases, red cell transfusion, as treatment options for aCKD.8 According to Wanner, most patients with aCKD who require substantial iron treatment will be prescribed an intravenous infusion by their nephrologist. However, they cautioned that while anaemia may be treated well in patients who regularly attend and adhere to hospital outpatient clinics, this applied to only a minority: “Nothing is stated in the general practitioner guidelines about referral of CKD patients due to anaemia. The only recommendation is for kidney function (eGFR falling below 30 mL/min/1.73 m2), so most patients are not treated.”
If treatment is received, then the means of monitoring its effects remain centred on laboratory testing, while measurements of QoL or other patient-reported outcomes are not routinely employed. There was strong consensus that while patient input on QoL would be highly beneficial for optimising treatment, applying standard questionnaires to quantify and track patient outcomes could create challenges. Wanner commented: “We do not listen any more. We do not have patient-reported outcomes. No QoL measurements. You can give patients a form to fill out while they are waiting, which works quite well, but what do you do with this information? There isn’t the time. In cardiology, there are heart failure nurses whose main job is to talk to the patients, but we do not have kidney failure nurses in nephrology.” In addition, Reast commented: “It’s really important that the QoL questionnaire is relevant to the individual, based on their previous/desired level of activity, and I know that some QoL measures are not.”
In a complementary approach, all three interviewees suggested that a checklist of simple questions about a patient’s life post-diagnosis/treatment, even something as simple as “How are you feeling?” could provide useful, qualitative information about their condition, and guide its management. Reast explained: “Then the connection could be made between what their haemoglobin’s doing, how they might be feeling, and what they might be able to achieve. Then it could be a lot better managed. The questions also serve as a reminder that physicians are willing to hear about what an individual is going through and how it affects them, and that there are answers.”
Impact of Treatment for Patients
The patient view was clear: optimal treatment of anaemia can be transformative. “If the treatment works well, then in only a few weeks you feel alive again,” said Gallego. “You have more energy, and even your physical appearance improves. You feel more confident, with greater self-esteem, and everything improves at the same time: relationships, work, hanging out with friends. Everything comes back into your life and you feel better, happy.” Reast was similarly positive about the effects of their anaemia treatment: “Physically and emotionally, I felt so much better, and was able to do much more. Prior to treatment, I couldn’t walk for a minute without needing to sit down and afterwards I was able to walk 3 miles slowly, while still on dialysis. My QoL completely changed, as did my energy for things, and that then impacted my view on life. Being able to do a few more of the things that I previously had to limit just makes it so much easier to have a positive mental outlook.”
Reast also gave an interesting insight on how effective treatment can in itself be a means of patient education: “I struggled with mental health, particularly linked to managing some of the aspects of CKD, so didn’t always do the best with compliance [with my erythropoietin injections]. Then, when it was all properly treated, I was quite annoyed at myself, because I felt so much better. Essentially, I had a bit of a wake-up call. Ultimately, I was putting myself in danger, not just in the short-term, but also long-term.” Thus, patients could benefit from being better informed about the consequences of non-adherence. Reast also emphasised that a non-judgemental and human-centred approach, looking at underlying needs and wants, would make it easier for patients to “buy into their treatment.”
SHARED DECISION-MAKING AND COMMUNICATION
While it is patients who are immersed in the daily burden of anaemia, it is the physician who currently dominates the treatment decision-making process. Patients may often express preferences over side effects or the administration route, but, according to Reast, altering dosing, for example, as a reactive response to such concerns does not constitute true “shared decision-making,” as it is not a two-way conversation. Current decision-making practices may also reflect a difference between patient and physician priorities. For example, in a European study of treatment preferences for anaemia of non-dialysis-dependent CKD, patients cited convenience of administration (mode/frequency), and an outcome of increased energy as important treatment attributes for which they would tolerate increased cardiovascular and gastrointestinal risk.6 In contrast, physicians prioritised clinical efficacy, and while symptom improvement, in particular increased energy, was widely acknowledged as being meaningful for QoL, only a minority recognised mode of administration as being most important to patients.6
These differences in viewpoint indicate that empowering patients to contribute to shared decision-making could be influential for the treatment of aCKD. Gallego considered the timescale of this approach: “To empower people you need education, not only information. Knowledge takes time to accumulate, so at [the point of diagnosis] I think it is almost impossible to enable real, shared decision-making, but over the years, with good communication with your nephrologist, you may be able to choose a better approach to improve your anaemia symptoms.” Reast described further advantages: “I think there would be a huge benefit of treatment decisions being shared because then you’re invested, not just a recipient; you understand the decision, the treatment, and the impact it can have on your life. However, not all specialists welcome patient input, and don’t think to bring in the patient as someone who can help control their condition, but I think that’s changing.” Indeed, Wanner’s view is that if a patient appears knowledgeable, then the physician is likely to be more attentive, and offer a more interactive discussion. This is also Reast’s experience, as through their own efforts in sourcing information, they have become better informed, and feel that their consultations now involve more shared decision-making: “I feel better able to have these conversations because I’ve gone out and found out information. I also learned through bitter experience, though obviously I wouldn’t want that to be the case for everyone.”
Asked for a physician’s opinion on whether shared decision-making would be a benefit to aCKD management, Wanner was clear: “Absolutely. Because I have learned that for CKD patients, other forms of intervention and greater care are much more valuable to the patient than ‘pill number 11’. Anaemia treatment would fall into the category of ‘greater care’, because if you talk to the patient about anaemia, many other things come to the fore. I think the communication between professional and patient is most important. Suggesting to the physician that investing in this process will, in the end, create a healthier patient to enter dialysis, and that a healthier patient will survive longer, may be the psychological approach needed to change practice.” Reast reinforced this point, explaining how an open conversation between patient and physician may help to deliver better treatment outcomes by uncovering potential issues and misconceptions. They admitted: “I never found injections easy to do, and I felt angry that there weren’t better, more suitable ways of treating anaemia. I think that if [this type of conversation] had been available to me, I would have said: ‘My haemoglobin hasn’t changed because I have not done my injections; it’s not necessarily because the treatment isn’t working, it’s because I’m struggling.’ Taking a couple of minutes to have this sort of conversation would pay off long-term, but I think it can be really difficult because of time pressures.”
IMPROVING EDUCATION AND MANAGEMENT AROUND ANAEMIA OF CHRONIC KIDNEY DISEASE
The experiences described here indicate a need for better education around aCKD, directed at both physician and patient. Reast commented: “[Education] definitely has a big impact, because the more aware I am, the more empowered I am to understand and provide benefits for myself and whoever’s looking after me, and to work together with my nephrology team. I can knowledgeably look at my haemoglobin level and know how it affects me as an individual. It allows me to look after myself better and reduce my visits to hospital emergency care, to the hospital rapid assessment units, and to my general practitioner for tests.”
Improving Physician Education
It was clarified that two main aspects of physician education need to be targeted in aCKD: clinical knowledge of anaemia, and understanding of patient impact and priorities through better communication practices. Concerning this first point, Wanner commented: “We have lost a lot of focus on anaemia in recent years because the erythropoietin-stimulating agent injectables were introduced, and so we knew how to manage patients, and the guidelines were forgotten. It’s very difficult to re-educate physicians, but my personal opinion is that if you bring the guidelines to their attention again, a few simple things, cornerstones, the two or three main statements, it may stimulate re-education.” Wanner thought that the advent of new oral medications may also bring attention back to the guidelines, and recognition of how anaemia may be better treated. Indeed, the arrival of new data and therapies have prompted an update to the KDIGO Guidelines for anaemia of CKD, last published in 2012, which is currently ongoing.8,14,15 However, Wanner felt that a bigger stimulation for change could come from the patients: “If they convey the impression that they are not being served well, it would make a greater impact than giving physicians the guidelines to study again,” they observed.
As a patient advocate, Gallego gave their view on educating physicians about patient priorities: “We need to educate professionals to tackle the anaemia symptoms in CKD, not only from the clinical perspective, but also around the humanistic burden that really matters to the patients. It is important to let them know that patients are really concerned about anaemia because it is affecting their QoL.” Reast added: “The more a physician understands the breadth of impact anaemia can have on someone’s life, the more seriously they’re going to take it.” Gallego commented: “Of course, we need to improve not only the knowledge of the professionals, but also their communication. If you ask the patients more, you will get a lot of feedback in a positive and a negative way. There are three views: one is the professional; the second is that of the patient organisation, patient advocacy; and the third is the patient testimonial, the regular people describing the reality. We joke that professionals give you the news, and patients give you the truth!” Encapsulating the situation, Wanner commented: “We need to encourage more patients to report, and we need to encourage more professionals to ask.”
Improving Patient Education
The key needs for patient education are to increase patient knowledge and understanding of the diverse potential symptoms of anaemia and its treatment, and thus empower them towards better communication and shared decision-making. However, the interviewees emphasised that for education to be most effective, it should be personalised. Gallego observed: “If all the information is given at the same time as the diagnosis, it is overwhelming. It takes years to gain knowledge and visit all the resources available, so information should be given repeatedly. Without any doubt, if you know all the possibilities and therapeutic options, you have the tools to choose better for yourself. Every patient is unique, and you need to personalise to every single patient, because what is an advantage for one may be a disadvantage for another.” From their physician’s view, Wanner said: “We need to educate patients to empower them, but I have so many different patients: those who are quite attentive and ask for their lab results, and others who are not interested. There are also many patients who just look through you, and you see in their eyes that they don’t understand. They just need to follow your advice, whereas others would benefit from [more information].”
Wanner also made the important point that it is not always about the education itself, but about engaging patients: “[For example], if patients are given their lab results, they may not immediately understand them, but they come back to you with questions, and then you can engage them. Another [approach] is translating results into something patients can understand, and which is important to them, such as daily functioning.” All three experts felt that improved awareness of the effects of anaemia in daily life, including on mental health, was central to improving patient education in aCKD. “A better awareness of all the different symptoms and how they will appear to you, as opposed to a long list of vague symptoms, is so helpful,” said Reast. “Having more education and conversations about anaemia is very important, and definitely anchoring it in everyday life. The better the person understands the impact of anaemia on their life if it’s left unchecked, the more seriously they’re going to advocate for themselves when they see their specialist.”
Materials for Patient and Physician Education
For patients, it was emphasised that educational materials should be up-to-date, credible, accessible, and bespoke for patient use, without complicated medical terminology. They should also be delivered through multiple channels (social media, websites, magazines, newspapers, apps, congresses, and meetings) and in a variety of formats (paper-based versus electronic) to create the greatest reach. In general, tools to help people make informed treatment choices were seen as useful, including a means of following and better understanding laboratory results in relation to daily life.
As a patient living with aCKD, Reast described the strengths of visual education with patient testimonies: “Especially when I was really tired, I didn’t like reading. Personally, I prefer videos: you get to see the person, you hear their voice, and it’s human. You can connect to that, and it has credibility. Hearing someone speak about their experiences, how important it is that anaemia is well managed, and how symptoms appear in everyday life, is so helpful because it just brings it to life.” In addition, Gallego and Reast agreed that distinguishing between the symptoms of CKD and the symptoms of anaemia should be a high priority for patient education. “Being able to both actively link anaemia to CKD but also to separate it out is important, so that people don’t think all symptoms are due to their CKD,” said Reast.
From the physician’s perspective, Wanner recommended a “refresher” of key points from the aCKD guidelines, while Gallego suggested a patient-adapted version of the guidelines could also be useful. In addition, Wanner highlighted a need for tools to aid physician–patient communication during consultations, and also noted: “Patients need to be encouraged to report more about their symptoms, even those they may feel embarrassed about, such as sexual activity, physical appearance, and relationships; whatever they feel is important to them.”
In terms of the contribution of patient organisations, Gallego spoke about the role of patient advocacy and the EKPF in delivering support and education: “We aim to help people understand CKD, and to claim equity and access for their treatments and their social rights. We also make sure that professionals and patients are always together at the round table for discussions. But the most important thing we do is to try and give hope. We say to patients that while CKD is tough, you can improve your life, your QoL, and your understanding, and we provide resources to empower them. Our ultimate goal is that they don’t need us at all.”
CONCLUSIONS: ALIGNING PATIENT AND PHYSICIAN AWARENESS OF ANAEMIA IN CHRONIC KIDNEY DISEASE
The three interviewees provided detailed insights into current awareness of aCKD, and related challenges. It was explained that dissecting out the symptoms of anaemia within CKD, and highlighting their impact on patients’ daily lives, is a priority that needs to be better addressed. Although there is some disconnect between physician and patient standpoints, it was felt that both parties require education in order to improve disease management and to address patient QoL. For physicians, this centres on refreshing their knowledge of diagnostic and treatment guidelines, and appreciating the humanistic burden of anaemia beyond clinical (haemoglobin) measures. For patients, education around the recognition and potential relief of anaemia symptoms is key, empowering them to make informed choices and be actively involved in their care. Facilitated by raised awareness and understanding, open communication and shared decision-making between patient and physician could bring substantial benefits to people living with aCKD (Figure 1).
Key takeaways
– Anaemia is a common, serious complication of CKD that raises morbidity and mortality risks, and presents a significant humanistic burden.
– Anaemia affects all aspects of daily living, impacting on physical and mental activities, relationships, and work productivity.
– With prompt recognition and diagnosis, anaemia can be effectively treated, but this requires greater awareness from both physician and patient.
– Physicians need to be better informed about recognising anaemia and realising the significant impact it can have on patients with CKD.
– Patients need more education to identify the symptoms of anaemia, empowering them to raise potential concerns, better understand and adhere to their treatment, and obtain symptom relief and improved QoL.
– More open communication between physician and patient would facilitate shared decision-making, and deliver optimal, personalised care.







