Meeting Summary
An integrated symposium, with an esteemed panel of experts, discussed the burden and treatment approaches for Clostridioides difficile infection (CDI). The session took place on 17th April 2023 as part of the 33rd European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) 2023 in Copenhagen, Denmark.
The session was co-chaired by Mark Wilcox, Head of Microbiology Research & Development and Infection Lead at Leeds Teaching Hospitals NHS Trust, UK, and Professor of Medical Microbiology at the University of Leeds, UK; and Anne Gonzales-Luna, Assistant Professor at the University of Houston College of Pharmacy, Texas, USA.
Sarah Tschudin-Sutter, Professor and Head Division of Hospital Epidemiology at the University Hospital Basel, Switzerland, explored the changing landscape for CDI, including the epidemiology and surveillance of infection, and approaches to diagnosis.
John Coia, Professor Emeritus at the Institute of Regional Health Research, University of South Denmark, and Honorary Research Fellow at Glasgow University, UK; and Esther Calbo, Professor and Head of Infectious Disease at the Hospital Universitari Mútua de Terrassa, Spain, gave an overview of the burden of recurrent CDI, dysbiosis in CDI, and the role in recurrent CDI, mortality risk, and the importance of targeting recurrence.
Calbo also presented an interactive case study that allowed audience members to engage and review the patient case presented. The faculty panel explored with the audience how to approach assessment and treatment to achieve the best outcome for the patient case.
Benoît Guery, Associate Professor Infectious Disease at the Centre Hospitalier Universitaire Vaudois, Switzerland, presented data from the EXTEND study, which explored extended-pulse dosing regimens of antibiotics (fidaxomicin) in CDI.
Introduction
Mark Wilcox
Clostridioides difficile (formerly known as Clostridium difficile and commonly referred to as C. difficile) is a Gram-positive anaerobic bacterium that causes CDI.1 It is a leading cause of healthcare-associated infections and is considered a global public health threat.1
Wilcox opened the symposium highlighting the epidemiological burden of CDI. In the USA, CDI is considered an urgent threat, with approximately 223,900 cases per year compared with approximately 124,000 CDI cases per year in Europe.2,3 Wilcox believes that these figures are underestimated due to attainment issues, and noted that C. difficile is associated with high mortality rates (3,700 deaths in Europe and 12,800 deaths in the USA each year).2,3 Wilcox emphasised the burden of CDI, stating mortality within 28 days post-diagnosis rates are higher than the recognised burden and mortality associated with meningitis. Approximately 25% of patients treated for CDI may experience recurrence, which can be due to relapse with the same strain or reinfection with another C. difficile strain, and up to 65% of these patients may experience further recurrences.4-6 Wilcox emphasised that C. difficile is “extremely good at finding weaknesses in a system.” Notably, also, selecting an optimal treatment is critical to ensuring patients do not experience CDI recurrence.
The Changing Landscape of Clostridioides difficile Infection
Sarah Tschudin-Sutter
Tschudin-Sutter commenced with a comprehensive overview of the global CDI epidemiology.7 In the USA, crude rates of healthcare-associated CDI have declined from 92.8 cases per 100,000 persons (2011) to 50.1 cases per 100,000 persons (2020), indicating the success of healthcare setting interventions.8,9 However, community-associated infections remain stable at 51.2 cases per 100,000 persons (2020) compared to 48.2 cases per 100,000 persons in 2011.8,9
Recent data shows that C. difficile was identified as the most common pathogen in patients with acute infectious gastroenteritis (32.2%) in the USA outpatient setting.10 In Europe, CDI ribotype (RT) distribution has changed. Toxinotype IIIb (027, 181, and 176) has declined in many European countries, but high prevalence rates were seen in Eastern European countries, the region with the lowest testing rate.11 Tschudin-Sutter highlighted that community CDI cases are often “undetected due to the absence of clinical suspicion,” accounting for three times more undiagnosed adults in the community compared with the hospital setting (approximately 111,000 compared with 37,000 cases per year in Europe, respectively).11 Tschudin-Sutter then compared the global epidemiology associated with CDI, where data is “lacking” or underestimated in many regions, however, remains an important cause of diarrhoea, with differing distribution of RT and sequence types.12-15
Tschudin-Sutter noted the COVID-19 pandemic had a varied impact on CDI rates, with reports of both an increase and decrease in incidence. In the southeastern USA, CDI incidence increased during the pandemic period (March 2020–March 2021) by 4.2% per month (95% confidence interval [CI]: 1.7–6.8; p=0.001; pandemic trend change rate ratio: 1.04 [95% CI: 1.01–1.07]), particularly in smaller community hospitals, “possibly due to staffing and resource constraints,” stated Tschudin-Sutter.16 In contrast, the Netherlands reported a lower annual incidence of CDI during the pandemic period (2020), which may be due to lower testing rates, but a higher percentage of severe cases, especially in the second wave of the pandemic (September 2020–January 2021).17 In severe cases, this increase was related to delayed community-onset CDI diagnosis (time to detection ≥8 days from start of symptoms).17 In the UK, overall CDI rates have generally declined (2007–2022) due to the success of interventions.18 However, a large increase in hospital- and community-onset CDI cases occurred during the COVID-19 pandemic (April 2021–March 2022), representing a 9-year high, and a 3-year consecutive increase.18 Tschudin-Sutter emphasised the importance of returning to conventional infection prevention and control practices, and building resiliency in such programmes in light of this data.19
Tschudin-Sutter highlighted the importance of the ‘One Health’ concept in managing CDI. The identification of possible sources is important for the understanding of CDI epidemiology. A multinational European study found that 22.4% of retail potatoes tested positive and may serve as a vector for introducing C. difficile spores into households where, if ingested, they could multiply in sensitive hosts.20 In terms of potential emerging C. difficile resistance, a study in Czechia found diverse C. difficile strains in waste- and surface-water samples, including a newly identified plasmid-mediated resistance to metronidazole, a drug used for the treatment of CDI.21 There are also reports of potential reduced susceptibility to vancomycin reported in Africa, the Middle East, and the USA.22–24 Tschudin-Sutter noted that “ongoing clarification” is needed to determine whether this is an emerging problem.
The Patient With Recurrent Clostridioides difficile Infection: Understanding Risk Factors and the Role of the Microbiome
John Coia and Esther Calbo
Coia gave an overview of the burden of recurrent CDI, with a focus on the gut microbiome, which normally protects against CDI through ‘colonisation resistance’. However, disruption of the gut microbiota increases susceptibility to recurrent CDI by allowing ingested C. difficile spores to germinate, multiply, and produce exotoxins.25 These elicit a profound inflammatory response leading to epithelial cell death and underlying connective tissue disturbance, resulting in characteristic features of CDI, such as colitis, colonic inflammation, and profuse diarrhoea (Figure 1).25
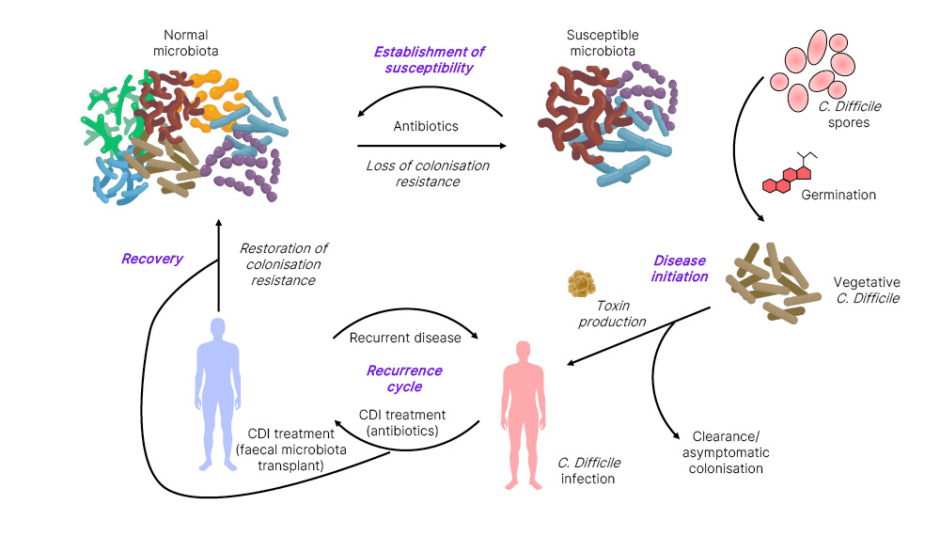
Figure 1: The cycle of recurrent Clostridioides difficile infection.25
Adapted from Britton and Young.25
C. difficile: Clostridioides difficile; CDI: Clostridioides difficile infection.
The most common cause of gut microbiota disturbance is antibiotic therapy.25 Although appropriate antibiotics targeting C. difficile resolve symptoms and restore the microbiota over many months, a significant minority of patients develop subsequent cycles of recurrent CDI with associated morbidity and mortality.25
The European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines define CDI recurrence as a renewed presentation of CDI within 8 weeks of the resolution of symptoms from the previous episode.26 Coia noted that “discriminating between relapse from reinfection is not routinely available in clinical practice;” however, multi-locus variable-number tandem-repeat analysis has identified 75% of first recurrences are due to relapse with the same strain.4 Whole genome sequencing studies have confirmed that the majority of recurrences, particularly early recurrences, are due to relapse, and some later cases also due to relapse.27 Widespread use of whole genome sequencing in clinical practice is expected to help differentiate relapse and reinfection.5
A systematic literature review of large-sized studies with more than 1,000 patients (n=27; of which 16 were from the USA) found overall CDI recurrence rates of 17% (range: 2–57%).1 The highest rates were seen in Canada (18%), the USA (17%), and Europe (UK [22%], Poland [22%], Germany [18%], and Spain [57%]).1 Coia indicated that the cut-off for a recurrent episode in most studies was ≤8 weeks after the first episode, and most only report overall recurrence rates without considering number of recurrences. The median recurrence rate from all studies was 17% (range: 0–64%).1 Recurrence rates were lower in community-associated than healthcare-associated CDI, possibly reflecting the younger age and lower exposure to healthcare facilities in this group.5
The European Centre for Disease Prevention and Control (ECDC) report for CDI from a multinational hospital surveillance (23 countries) between 2016–2017 with more than 18.3 million patient admissions and over 109 million patient days, identified a 6.4% recurrence rate (n=2,439 out of 37,857), with a crude incidence density of 0.22 recurrent CDI cases per 10,000 patient-days.28 Recurrence was most common in tertiary hospitals, and twice as likely to have a complicated course of infection than non-recurrent cases (25% versus 14%; p<0.0001), and higher mortality related to recurrent CDI cases (31% versus 21%; p=0.003).28 Up to 35% of CDI cases recur, with 20% recurring after a single episode, 40% after two episodes, and 65% after three episodes.4,5 Recurrent CDI is associated with increased morbidity, mortality, and inpatient hospital costs compared with non-recurrent CDI.29-31
The ESCMID guidelines highlight several risk factors associated with recurrent CDI,26 including age >65 years (relative risk: 1.63 [95% CI: 1.24–2.14]; p=0.00050);32 prior CDI episode (particularly previous severe CDI); healthcare-associated CDI (admission within the last 3 months); concomitant non-CDI antibiotic use after diagnosis (relative risk: 1.76 [95% CI: 1.52–2.05]; p<0.00001);32 and gastric acid suppression, such as proton-pump inhibitor (PPI) use, during or after CDI diagnosis (22.1% versus 17.3% without; odds ratio: 1.52 [95% CI: 1.20–1.94]; p<0.00100).33 Other risk factors include severe underlying disease, such as inflammatory bowel disease, renal insufficiency, inadequate immune response to C. difficile toxins A and B, and virulence of the infecting strain.26,34 Narrow-spectrum antibiotics such as fidaxomicin or vancomycin are associated with a lower rate of CDI recurrence, with fidaxomicin having a lower recurrence rate compared to vancomycin.35,36
Whilst these risk factors are helpful, Coia noted that “there are no specific tests or markers that accurately predict patients’ likelihood of developing recurrent CDI,” although age >65 years is considered the most important risk factor.26 This is an important unmet need not only for the prognosis of these patients, but also for helping better targeting of therapeutic options.
The Role of Dysbiosis in Recurrent Clostridioides difficile Infection
Recurrence of CDI is likely caused by a combination of microbiome disruption factors, including failure to re-establish the colonic microflora, the presence of C. difficile spores in the intestines, and a suboptimal host immune response to the infecting organism and its toxins.37
Dysbiosis, as generated by broad-spectrum antibiotics, is an imbalance in gut microbiota, characterised by reduced microbiota diversity, an increased proportion of other species (e.g., Escherichia coli, Proteus mirabilis, and Enterococcus faecalis), a loss of resistance to colonisation, and an increase in pro-inflammatory cytokine synthesis, and has been proposed as a key factor in CDI recurrence.35,38 The mechanisms through which gut microbial dysbiosis drives CDI are complex and not fully understood; however, the gut microbiota is mainly composed of Firmicutes (64%) and Bacteroidetes (23%). Gene sequencing studies have shown alterations in microbial composition of Bacteroidetes and Firmicutes, and marked decreased species diversity in patients with recurrent CDI, as well as in patients with non-C. difficile diarrhoea compared with healthy controls.38-40 Calbo discussed the molecular mechanisms underlying dysbiosis in CDI, including the role of intestinal bile acid composition and spore germination, gut microbiota competition for nutrient niches inhibiting C. difficile growth, and zinc and other elements facilitating metabolic adaptation of C. difficile.
Alterations in the gut metabolome and expansion of antibiotic-resistant enterococci alter the gut metabolic environment and reprogramme C. difficile metabolism by a parallel process of nutrient restriction and cross-feeding, and may also play a role in CDI pathogenesis.41,42
Calbo stated that there are more than 2,000 different bile acids, so a simplification of the process is that primary bile acids (cholate derivates) promote C. difficile spore germination (with co-germinants such as amino acids, calcium, and glycine) while 7α-dehydroxylation by gut microbiota to generate secondary bile acids inhibits spore germination and vegetative C. difficile cell growth, depleting the pool of primary bile acids.43,44 Intestinal bacteria that mediate 7α-dehydroxylation have been shown to be protective against CDI in a mouse model.45 Furthermore, a Phase II, double-blinded, placebo-controlled study investigating stool samples (n=113) from patients with recurrent CDI (N=27) who were administered a microbiota-based live biotherapeutic, showed a reduction in dominant primary bile acids and concurrently increased secondary bile acids, which correlated with “clinical cure.”46
Regarding nutrient competition, there are many important molecules, including gut microbial-derived short chain fatty acids, such as propionate, acetate, and butyrate, which play a role in maintaining intestinal barrier integrity, inhibit pro-inflammatory cytokines, and serve as an energy source for colonic epithelium cells, which are associated with CDI resistance.43 The gut microbiota competes for nutrients with C. difficile by depleting carbohydrates, amino acids, glycine (a co-germinant for spores), and cholesterol (by producing coprostanol).43,47 C. difficile has adapted its metabolism to transport and uptake metal ions, such as zinc sequestration by calprotectin (Figure 2), and uses mannitol as a primary nutrient, which is abundant in post-antibiotic environments.47 Furthermore, C. difficile also produces bacteriostatic compounds such as p–cresol (a tyrosine metabolite) and sorbitol.43
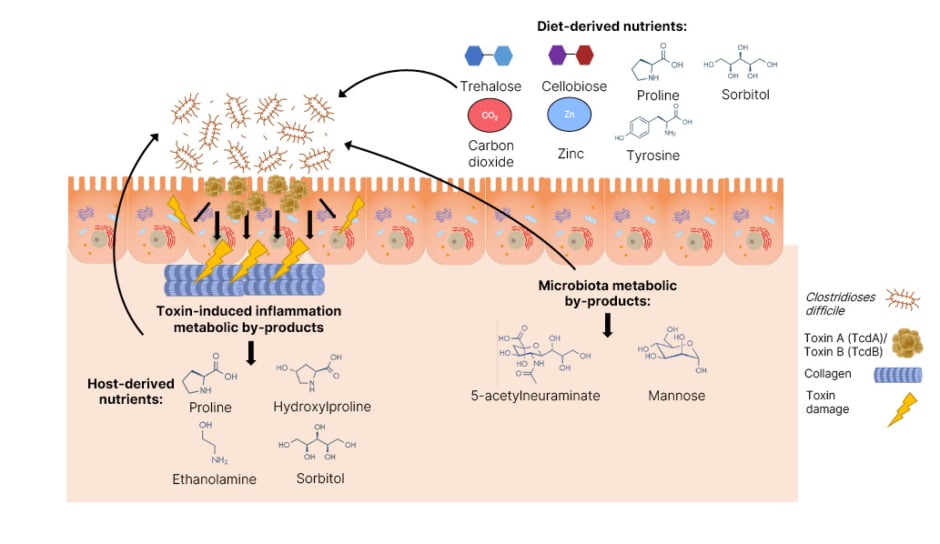
Figure 2: An overview of the nutrients Clostridioides difficile utilises and their origin during
infection of the gut.46
Adapted from Marshall et al.46
C. difficile: Clostridioides difficile.
Understanding the Risk Factors and Outcomes of Recurrent Clostridioides difficile Infection
Calbo emphasised the importance of targeting recurrence to improve CDI outcomes, a composite of recurrence of CDI, refractory CDI, severity, and mortality risk.48 However, Calbo stated studies on CDI mortality are “scarce and vary widely in methodology.” Some studies describe rates of poor mortality outcomes related to specific RTs, such as BI/NAP1/027 and 078.48
Calbo noted that CDI mortality risk factors are similar to those for CDI recurrence, including age, number of comorbidities, cancer, and BMI.49 Poor outcomes in CDI result in 14.0–25.0% recurrence, 4.0–20.0% refractory, and approximately 6.0% mortality, with in-hospital mortality ranging from 8.0–37.2%.48 Moreover, outcome drivers include C. difficile virulence factors, host factors such as age and malignancy, and treatment.49,50 A retrospective single centre cohort study on almost 4,000 patients with CDI found that recurrent CDI is associated with an increased risk of death at 3 and 6 months; and a UK study with 6,682 patients with CDI, including 1,140 patients with recurrent CDI, found an increased risk of mortality and complication at 12 months.29,30 Additional studies show that CDI is characterised by a high delayed and unrelated mortality rate (18% at 75 days), associated with age (>65 years), comorbidity, and faecal incontinence.51 Calbo investigated CDI in patients with cancer, and found a higher risk of CDI recurrence (13%) and mortality (27%), particularly late mortality (3 months after initial episode: 13%).52 Calbo concluded that in select populations (the elderly, patients with cancer, and patients with recurrent CDI), delayed mortality rates may be higher than early mortality rates.
Clostridioides difficile Infection in Practice: Interactive Case Study
Expert Panel
During an interactive session involving the audience by use of a mobile application, Calbo outlined the case of an 86-year-old female living in a skilled nursing facility, with a history of chronic oedema and kidney disease, hospitalised 2 months prior with heart failure, where they were diagnosed with gastroesophageal reflux disease, and started on a PPI. The patient had developed cellulitis 20 days prior, without systemic signs of infection, and was treated with antibiotics (oral clindamycin 300 mg four times daily [QID]). The patient subsequently developed abdominal pains and diarrhoea approximately 1 week after completing the course of antibiotics, and tested positive for CDI.
The panel and audience, consisting of participants from a wide range of countries (such as Germany, Italy, Spain, the UK, and the USA) and specialities (including infectious disease specialists and clinical microbiologists), agreed (93.2%) that the patient was at risk of recurrence due to age (>65 years), prior antimicrobial exposure and PPI use, and environment (living in a skilled nursing facility and prior hospitalisation within the past 3 months).26,32-34
For the initial CDI, 63.5% of the audience selected fidaxomicin (200 mg twice daily [BID] for 10 days) as the best therapy for this patient, while 30.4% selected vancomycin (125 mg QID for 10 days), and 6.1% chose metronidazole (500 mg three times daily for 10 days). Wilcox emphasised that guidelines “no longer recommend” metronidazole as a first-line therapy for primary CDI, whether there is a risk of recurrence or not.26 They further emphasised the importance of adherence to the guidelines to improve outcomes for patients, with fidaxomicin and vancomycin being standard of care. Fidaxomicin should be used as first-line, followed by vancomycin if fidaxomicin is not available. Metronidazole is no longer recommended.26
Despite receiving fidaxomicin for 10 days for the initial CDI, the patient experienced two subsequent recurrent episodes, approximately 4 weeks after completing the first course, and approximately 3 weeks after completing the second course (fidaxomicin [200 mg BID for 10 days] plus bezlotoxumab infusion [10 mg/kg administered on Day 6 of fidaxomicin]). Calbo confirmed the patient was toxin positive in the last recurrence, with symptoms of pain and fever.
The panel agreed on faecal microbiota transplantation (FMT) as the next step despite acknowledging the risks, with Wilcox advising caution for use of FMT prior to recurrent CDI, and before exploring optimal and alternative treatment pathways (Figure 3).26 Gonzales-Luna questioned that perhaps the first episode of CDI could have been treated with fidaxomicin plus bezlotoxumab. Guery confirmed that this would have been an option, but there was no strong data supporting it. This lack of data may also raise some cost concerns. Coia emphasised the importance of diagnostics and identifying risk factors for recurrent CDI to select the appropriate treatment, and Wilcox highlighted the need to determine where current and emerging treatments fit in the therapeutic pathway.
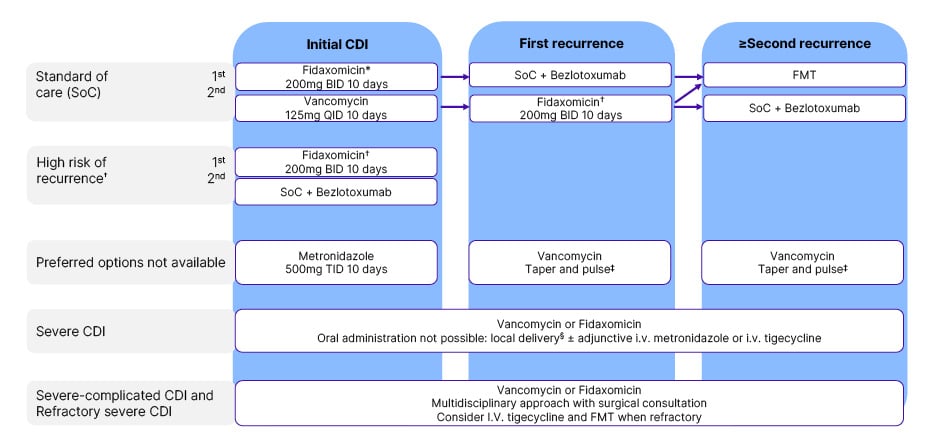
Figure 3: European Society of Clinical Microbiology and Infectious Diseases (ESCMID)-suggested
treatment recommendations.26
*Risk stratification for risk of recurrence may be applied for selective use of fidaxomicin in case of limited access or resources.
†Consider extended fidaxomicin: 200 mg BID on Day 1-5, 200 mg q48h on Day 7–25. Most important risk factor for recurrence is age >65–70 years. Additional risk factors to consider are healthcare-associated CDI, prior hospitalisation ≤3 months, prior CDI episode, continued non-CDI antibiotic use, and PPI therapy. The risk of recurrence is assumed higher with more risk factors present.
‡Vancomycin taper and pulse: 2 weeks 125 mg QID, followed by 1 week 125 mg BID, then 1 week 125 mg qd, then 1 week 125 mg q48h, and finally 125 mg q72h for 1 week.
Rectal or nasoduodenal delivery.
Adapted from Van Prehn et al.26
BID: twice daily; CDI: Clostridioides difficile infection; FMT: faecal microbiota transplantation; IV: intravenous; qd: once daily; QID: four times daily; q48h: administered at 48-hour intervals; q72h: administered at 72-hour intervals; SoC: standard of care; TID: three times daily.
Extended Dosing in Clostridioides difficile Infection: EXTEND Study
Benoît Guery
Guery discussed the use of fidaxomicin, a narrow-spectrum macrocyclic antimicrobial, for treating CDI, with a focus on the extended dosing approach.35 Fidaxomicin selectively targets C. difficile by inhibiting RNA polymerase, while having minimal effects on gut commensals.35 Guery suggested that fidaxomicin preserves the gut microbiota compared with broad-spectrum antibiotics, and “reduces the recurrence of CDI.”36,53 This may be partly due to gut microbiota such as Proteobacteria or Bacteroidetes lacking the fidaxomicin binding site.34
In an in vitro study using a human chemostat gut model, fidaxomicin first-line was effective in reducing the total viable count of C. difficile, spore counts, and cytotoxin titre compared with vancomycin and metronidazole.54 Alternative dosing regimens, including extended (20 days with 200 mg/L BID) and tapered-pulsed dosing (5 days 200 mg/L BID, followed by 20 days 200 mg/L once every other day) were effective in reducing C. difficile and toxin detection with no recurrence, while sparing microbiota.54 Pulsed or tapered regimens enabled greater recovery of Bifidobacteria compared with the extended regimen.54 Guery believes that this could be interesting in the gut healing process.
The EXTEND Phase IIIB/IV study is an open-label, randomised, multinational controlled trial conducted in 86 centres across 21 countries.55 Patients >60 years old (N=364) were administered either an extended-pulsed fidaxomicin regimen (n=177; 200 mg BID on Days 1–5, followed by 200 mg once daily on alternate days over Days 7–25) or vancomycin (n=179; 125mg QID on Days 1–10).55 The primary endpoint was sustained clinical cure 30 days after the end of the treatment (Day 55 for fidaxomicin and Day 40 for vancomycin), with follow-up at Day 90.56 Approximately 58.1% of participants were female, most had non-severe CDI (63.5%), 78.9% of participants had not experienced a previous CDI occurrence in the 3 months prior, and approximately 72.0% received antibiotics for conditions other than CDI, indicating they were high-risk for CDI recurrence due to age (>60 years) and systemic antibiotic use.26,55
Although there was no difference in clinical cure, a significant decline in recurrence was observed between Day 40 and 55 (-15% and -14%, respectively; p<0.0001).55 Extended-pulsed fidaxomicin was found to be superior to standard-dose vancomycin for sustained clinical cure of CDI, demonstrating that efficacy was preserved in patients with a high risk of recurrence.55 The extended-pulsed fidaxomicin regimen is approved for use in Europe.56,57 Subgroup analysis identified similar efficacy in the extended-pulsed fidaxomicin regimen with a preserved rate of sustained clinical cure regardless of risk factors, such as age (≥60 years), cancer diagnosis, CDI severity, prior CDI episodes, or infection with RT027.58 Extended-pulsed fidaxomicin showed sustained clinical response rates of 74% at 30 days (n=34 out of 46) and 61% at 90 days (n=28 out of 46) in 46 high-risk patients with multiple CDI recurrences (57% ≥65 years old; 39% using PPI; and a mean of 3.5 previous CDI episodes) who failed tapered vancomycin treatment (75%).59
Guery proposed future research to address limitations of the EXTEND trial to consider the use of randomised control trials versus conventional administration, including patients under 60 years old. They suggested comparing extended fidaxomicin with vancomycin and bezlotoxumab as standard of care, and versus fidaxomicin pulsed approach. Additionally, Guery suggested the need for data on multiple recurrence, especially in cases where FMT is not available.
Question and Answer Session
Gonzales-Luna asked Calbo if fidaxomicin resistance was tested in the patient case, and Calbo responded that they did not, as there is not currently “a problem.” Tschudin-Sutter identified the importance of routine surveillance for vancomycin resistance, and Coia emphasised the importance of conducting susceptibility testing properly and recommended “reference laboratories do monitor isolates for the potential of resistance for fidaxomicin,” while Wilcox supported the need for ongoing surveillance of minimal inhibitory concentrations.
The panel were asked about retesting protocols post-CDI infection. Coia said that “waiting 28 days from positive CDI cases is too long,” while Guery and Calbo indicated in their clinical practice testing only occurs in symptomatic patients (i.e., those with diarrhoea).
Regarding the importance of dysbiosis and the potential role of diet and foodstuffs, Coia and Wilcox called for further understanding of this, as well as the role of One Health in C. difficile transmission.
Regarding treatment approaches, Coia recommended following ESCMID guidelines for first-line therapy and dosage approach, with fidaxomicin (200 mg BID for 10 days) as standard of care, or vancomycin (125 mg QID for 10 days) when not available (Figure 3).26 Calbo recommended that the more risk factors a patient has for CDI recurrence, fidaxomicin plus bezlotoxumab should be used. Gonzales-Luna asked whether tapered or pulsed vancomycin or fidaxomicin could be used in cases where other options are unavailable or unsuitable, and Guery agreed that data supports the use of these approaches, but highlighted gaps for multiple recurrence.








