INTRODUCTION
Asthma is a chronic inflammatory disease where >100 powerful inflammatory mediators are associated with airway hyper-responsiveness, mucus hypersecretion, activation of fibroblasts, hyperplasia, and hypertrophy of airway smooth muscle.1 Cytokines are involved in this inflammation. The cells that produce cytokines include B cells, T cells, dendritic cells, natural killer cells, cytotoxic T cells, T helper cells (Type 1 and 2), endothelial cells, mast cells, plasma cells, progenitor cells, bone marrow cells, thymus cells, and tumour cells, together with fibroblasts, leukocytes, monocytes, and macrophages. Type 2 T helper cells produce granulocyte macrophage colony-stimulating factor (GM-CSF) and interleukins (IL), including IL-4, IL-5, IL-9, IL-13, IL-25, IL-31, and IL-33, which are responsible for chronic eosinophilic inflammation, the type of inflammation that is characteristic to allergic diseases such as asthma. This complex pathophysiological process leads to the clinical manifestation of asthma with recurrent episodes of wheezing, breathlessness, chest tightness, and coughing.1-4
Severe asthma is defined as “asthma which requires treatment with high-dose inhaled corticosteroids (ICS) plus a second controller (and/or systemic corticosteroids) to prevent it from becoming symptom control, frequent, severe, and serious ‘uncontrolled’ or which remains ‘uncontrolled’ exacerbations, and airflow limitation. If patients despite this therapy.”5 Uncontrolled asthma is do not use preventive anti-asthma treatment, defined as at least one of the following: poor it can cause irreversible airway remodelling.5
OBJECTIVES
The aim of this study was to determine the effect of adding montelukast to combined therapy (ICS/long-acting beta-agonists [LABA]) in patients with severe uncontrolled asthma by analysis of the serum level of IL-5, IL-13, and eosinophils, and the symptom score.
METHODS
In this study, we included 29 patients that were treated with ICS/LABA (500/50 μg twice daily) plus montelukast (10 mg daily). In each patient, we measured the serum levels of IL-5 and IL-13 by the enzyme-linked immunosorbent assay (ELISA) method at the Institute of Immunobiology and Human Genetics, Faculty of Medicine, Ss. Cyril and Methodius University of Skopje, Skopje, Macedonia. The number of eosinophils was obtained using visual examination of a peripheral blood smear at The University Clinic for Haematology, Skopje, and by assessing symptom scores with 5-point Likert scale of breathlessness at the beginning and after 6 months of therapy. The reference values for IL-13 are 0–6.9 pg/mL, for IL-5 are 0 pg/mL, and are 0.58–0.66% for eosinophils.
RESULTS
The results were statistically analysed according to the Wilcoxon Paired Test, using a significance value of p<0.05 and a high significance value of p<0.01. The obtained results of IL-5, IL-13, and value of eosinophils showed that the levels before the start of therapy were much higher and treatment of ICS/ LABA plus montelukast reduced these values with statistical significance (IL-5: Z=4.64; p=0.000004; IL-13: Z=4.7; p=0.000003; eosinophils: Z=2.99; p=0.0028; p<0.05). Symptom score results also showed statistically significant improvements (Z=4.54; р=0.000006) after 6 months of therapy (Table 1).
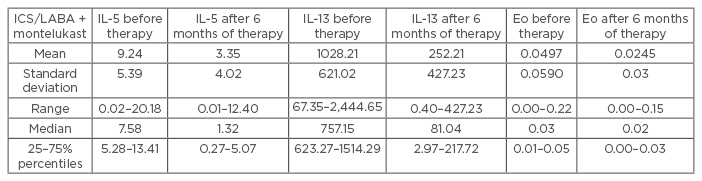
Table 1: The descriptive statistics for IL-5, IL-13, and eosinophils in asthma patients treated with ICS/LABA plus montelukast.
IL: interleukin; ICS: inhaled corticosteroids; LABA: long-acting beta-antagonists; Eo: eosinophils.
CONCLUSION
Combination therapy with ICS/LABA represents the gold standard in the treatment of asthma. It is a safe and effective treatment that is recommended by the Global Initiative for Asthma in the treatment of uncontrolled, severe, and persistent asthma in Steps 3 and 4 of treatment.1 Combination high-dose of ICS/LABA generally provides additional benefit for patients, but it is well recognised that not all patients will achieve well-controlled asthma despite an appropriately high dose of ICS or ICS/LABA combination therapy. In such patients, there is a need for additional add-on therapy, such as treatment with a leukotriene receptor antagonist (Figure 1). The addition of this treatment to patients whose asthma was considered to be insufficiently controlled resulted in a significant clinical improvement in asthma control, pulmonary function, and quality of life, and the dose of ICS/ LABA was able to be reduced.6-9 This suggested that these markers of inflammation are important for monitoring disease evolution and success of therapy in patients with asthma.10
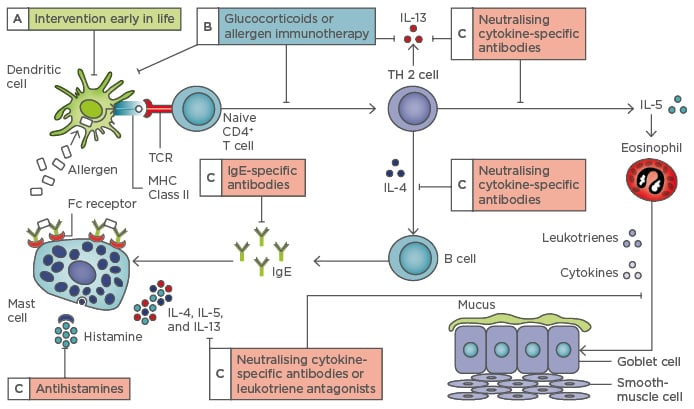
Figure 1: The allergic pathway and potential points of intervention.
TCR: T cell receptor; MHC: major histocompatibility complex; IL: interleukin; TH 2: T helper cell Type 2; Ig: immunoglobulin.
Adapted from Hawrylowicz CM, O’Garra A.4






