Abstract
Food allergies, which are T helper cell Type 2 aberrant responses of the immune system to food proteins, are increasing. Environmental factors, including food contaminants, are often mentioned to explain this increase. Heat treatment of food induces the Maillard reaction, a non-enzymatic reaction between reducing sugars and free amino groups of proteins or free amino acids. This leads to the genesis of neoformed compounds, including advanced Maillard reaction products (also called dietary advanced glycation end-products [AGEs]). Infant formulas are very sensitive to the Maillard reaction because of their high content of lactose and proteins and their long shelf life. The dietary AGEs content is particularly high in hydrolysed infant milk. Among dietary AGEs, Nε-carboxymethyllysine is the main form in milk. An increasing number of studies show potentially deleterious effects of dietary AGEs, including inflammation genesis. These effects seem to be in a great part dependent on the receptor of AGEs (RAGE). RAGE is present on immune cells and studies have shown that RAGE is involved in T helper cell priming, proliferation, and differentiation. Moreover, there is increasing evidence that the Maillard reaction enhances the allergenicity of proteins. All these data indicate a potential role of dietary AGEs in allergies. Nevertheless, the impact of dietary AGEs on the immune system favouring the T helper cell Type 2 profile and consequently predisposition to develop allergy is poorly documented and needs further investigation.
INTRODUCTION
The incidence and prevalence of food allergies have dramatically increased in recent decades, especially in children. The prevalence varies from one country to another but is particularly high in countries with Western lifestyles.1-4
Allergy is a hypersensitivity reaction with specific immunologic mechanisms inducing objectively reproducible symptoms initiated by exposure to a substance, the so-called allergen, at a dose tolerated by a healthy person.5 There are two distinct phases. The first phase, without clinical manifestations, is the allergen sensitisation phase. This leads to the activation and proliferation of a particular population of lymphocytes, the T helper Type 2 (Th2) cells. Th2-cytokines (interleukin [IL]-4, IL-5, etc.), will promote the production of allergen-specific immunoglobulin (Ig)Es and the recruitment of inflammatory cells (eosinophils, mast cells, or basophils).6 The second phase is the effector phase. Upon further contact with the allergen, the interaction between the allergen and IgEs on target cells, such as mast cells, results in the release of mediators, such as histamine. Induced symptoms vary from moderate reactions, such as eczema, to severe reactions, such as oedema or anaphylactic shock. Atopy is an individual and/or familial genetic predisposition to develop allergies with enhanced IgE-mediated responses to common allergens.5
Breastfeeding is often recommended up to the age of 6 months to reduce the risk of developing an allergy. However, beyond the child’s sixth month, the majority of parents use infant formulas made from cow’s milk. As a consequence, one of the first allergies in children is cow’s milk allergy (CMA). Thus, many parents use hypoallergenic infant formulas with partially hydrolysed proteins or extensively hydrolysed proteins, especially in atopic families, in order to limit the risk of developing CMA.7,8 However, their preventive action on the development of other allergies is not clearly demonstrated since the literature presents contradictory evidence.9-13
CMA is often only the first step in the ‘atopic march’ (or allergic career). If CMA is often relieved before the age of 10 years, the atopic child may become sensitised to other allergens and may develop new allergies, which will, most often, persist into adulthood. For example, 50% of children who have had CMA will develop another food allergy and 50–80% of them will develop allergy against inhalants.10,14
While the predisposition to develop an allergy is partly determined genetically, environmental factors (including diet) seem to play a very important role in the onset and development of allergies, especially in young children whose biological functions are immature.15 Several hypotheses have been proposed to explain the increased incidence of allergies, including the reduction of viral or bacterial exposure (hygiene hypothesis), changes in the composition of the gastrointestinal flora, or the presence in the food matrix of compounds that could modify susceptibility to develop an allergy.16 Since the industrial preparation of infant formula can generate neoformed compounds by glycation of milk proteins, formula-fed babies could be exposed to them, and in particular to advanced Maillard reaction products (advanced glycation end-products [AGEs]).
DIETARY ADVANCED GLYCATION END-PRODUCTS IN INFANT FORMULAS
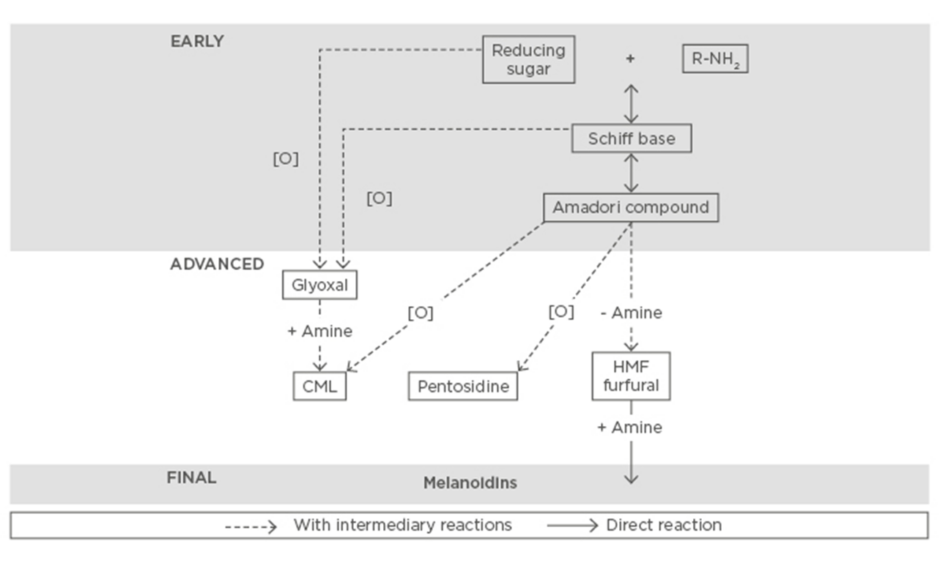
Heat treatment of food ensures its safety and extended shelf life. However, due to this treatment, several reactions occur and give rise to neoformed compounds genesis. In dairy products, the Maillard reaction is predominant among these reactions. This non-enzymatic reaction occurs between reducing sugars and free amino groups of protein or free amino acids. This reaction takes place in three steps (Figure 1). The initial step (Early) leads to the formation of Amadori products. This intermediate product (Advanced) can be then transformed into advanced Maillard reaction products (MRPs), also called AGEs, which are chemically more stable. The final step of the Maillard reaction (Final) can lead to the formation of brown polymers, called melanoidins.17
Figure 1: Main steps and pathways of the Maillard reaction.
CML: Nε-carboxymethyllysine; HMF: 5-hydroxymethylfurfural.
Adapted from Hodge.17
Dairy products contain very few free amino acids,18 with the exception of hydrolysed infant formulas. The Maillard reaction is favoured in milk because of its high content of lactose and proteins, such as caseins or serum proteins, that are rich in lysine. They carry amine functions that are involved in the Maillard reaction. The most predominant form of Amadori products is lactulosyllysine, which results from reactions between lactose and lysine.19 During prolonged heating, these Amadori products are transformed to several types of MRPs, whose main form is Nε-carboxymethyllysine (CML).19
Among the different forms of milk, infant formulas are those with the highest levels of CML (Table 1).20-22 CML levels of infant formula are ≤45-times higher than in highly sterilised (ultra high temperature) milks and ≤83-times higher than breast milk.23 The amount of CML in breast milk is dependent on the diet of the breastfeeding mothers, since CML does pass through breast milk, but is usually low.23 By contrast, the long shelf life of infant milk, and the often high iron and lactose content, makes it particularly sensitive to the Maillard reactin.24 Moreover, the highest CML content is measured in hydrolysed infant milk.21 This partial or total hydrolysis of these milk proteins leads to an increased proportion of free lysines and other amino acids, which will then actively participate in the Maillard reaction during heat treatment.
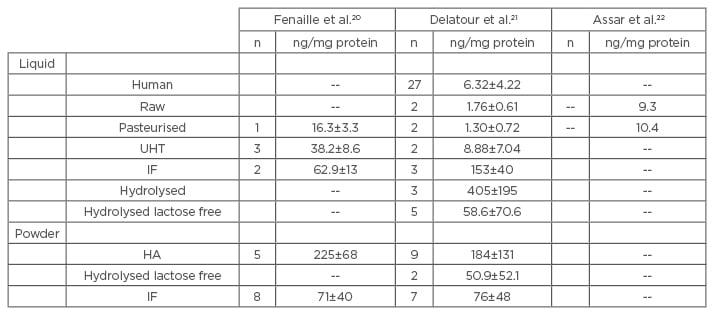
Table 1: Comparison of measurements of CML in different milks.
n=number of samples tested. CML levels are expressed in ng/mg protein.
UHT: ultra high temperature (highly sterilised); IF: infant formula; HA: hypoallergenic; CML: Nε-carboxymethyllysine.
DIETARY ADVANCED GLYCATION END-PRODUCTS AND HEALTH
AGEs were first described as endogenous compounds whose concentration increases with age. They form through an in vivo process, referred to as ‘non-enzymatic glycosylation’ or ‘glycation’. Their presence in excess has been described in age-related diseases, such as metabolic disorders, atherosclerosis, and Alzheimer’s disease.25 It has been shown that dietary AGEs, also called MRPs, contribute significantly to the systemic burden of AGEs.26 These compounds are chemically the same and mainly come from glycation. Furthermore, an increasing number of studies show potentially deleterious effects of dietary AGEs, although the results may be divergent due to the great variability of the forms and contents of AGEs depending on the food.27,28
Dietary Advanced Glycation End-Products and Gut Homeostasis
The host microbiota is a key component of gut homeostasis since it contributes to its physical protection, metabolism of food (e.g. short chain fatty acids production), and synthesis of new compounds (e.g. vitamins, neurotransmitters) but also exerts strong interactions with the mucosal and immune cells.29 This interaction with the host is a long process that starts mainly at birth and necessitates some training from either side to reach an almost perfect regulation. However, under some conditions, by modifying the Th balance, this cross communication is altered and the microbiota profile becomes altered. One of the conditions at the origin of such modifications is the food matrix. The composition of the food matrix conditions the gut microbiota profile, which adapts to it. This changes the type of interaction between the host and microbiota, which has two main consequences: the production of potentially antigenic substances and/or facilitation of increased numbers of potentially pathogen micro-organisms, including gammaproteobacteria, which are detrimental for lactobacilli and bifidobacteria.30
As stated above, the Maillard reaction results in increasingly complex compounds whose effects on health are equivocal. This also applies for their interaction with the intestinal microbiota. From the literature, it has been indicated that Amadori products (e.g. fructoselysine) are poorly absorbed but metabolised by some bacteria.31 As they are generally ingested in high quantities, we may then suggest that they influence the composition (density and diversity) of the gut microbiota. By contrast, as CML is known to be rapidly absorbed and transported into the blood circulation, it will not be used by the intestinal microbiota. At last, due to their chemical composition, most of high molecular weight MRPs (e.g. melanoidins) are likely to escape the upper gastrointestinal tract and may be more susceptible to be metabolised by the microbiota32 as they behave as prebiotic fibres.
Formula-fed infants present 46% higher plasma CML levels and higher CML (60-fold) urinary excretion than breast-fed infants.33 This is as CML absorption and urinary excretion are mostly linked to the level of CML in the food matrix (dietary intake), which is higher in formula than in breast milk, in which low levels of CML are detected.23
Free MRPs are not substrates for the intestinal lysine transporters.34 However, when bound to small peptides, some MRPs may translocate into epithelial cells via di and tri-peptides transporters (PEPT1). In general, the longer MRPs are peptide-bound during intestinal digestion, the more hydrophobic they are and there is a higher chance of their appearance in the circulation. Then, once they have translocated into the epithelial intestinal cell, they are submitted to intracellular proteolysis. While glycated amino acids with polar or charged chains (e.g. CML) remain trapped inside the cell, glycated amino acids with unipolar side chains (e.g. maltosine, pyrraline) can pass through the membrane.34-36 Their outcome is not clearly established and needs further investigation.
However, it is well understood that biologically formed AGEs bind to a plasma membrane receptor of AGEs (RAGE). This receptor is highly expressed by several types of cells, including immune, neuron, lung, and heart cells. Because it belongs to the immunoglobulin superfamily, once activated, it may initiate intracellular pro-inflammatory pathways, such as Ras/MAPK and JAK/STAT. These pathways often converge to nuclear factor-kappa B (NF-κB) activation and correlate to tissue damage by activating pro-inflammatory cytokines secretion.37-39 It is not clear whether these pro-inflammatory pathways are also involved in intestinal epithelial cells. From our preliminary in vitro data, we observed that, in physiologic doses (amount possibly found in food) CML seems not to alter the Caco-2 epithelial monolayer, since the trans-epithelial electric resistance (an indicator of intestinal permeability) is maintained after 24 hours of exposure. Moreover, from these data we did not measure any expression of RAGE in the absence, as well as in the presence, of CML at the dose used. However, we have not measured the amount of intracellular CML in the Caco-2 cells after their exposure to MRPs (unpublished data).
Dietary Advanced Glycation End-Products and Inflammation
Dietary AGEs may predispose individuals to inflammation, which plays a major role in the development of chronic diseases (for review40). Thus, dietary AGEs such as CML are suggested to participate in metabolic disorders41 and cardiovascular dysfunctions.42 As mentioned above, consumption of dietary CML is correlated with circulating CML levels but also with an increased release of biomarkers of the inflammatory reaction and/or oxidative stress in both humans and animals.43-45 By contrast, it has been shown that acute exposure to dietary CML may not influence inflammation46 and that some forms of CML are unable to activate an inflammatory response.47 As mentioned previously, arguments in favour of its participation in the inflammatory reaction are that RAGEs are present on immune cells (mononuclear phagocytes, dendritic cells, T cells) and that cells expressing high levels of RAGE are often close to areas in which AGEs are abundant.48-50 Their role in the regulation of the immune response has been studied in several in vitro and in vivo models.
Activation of immune cells, such as dendritic and TCD4+/TCD8+ cells, induce increased RAGE expression and this increase is more sustained in the presence of a RAGE ligand.51 Furthermore, Dumitriu et al.52pointed out that, in both human and mice in vitro models, neutralisation of RAGE reduces the maturation of dendritic cells. This in turn present a lower secretion of IL-12 and a lower activation of the intermediate signalling pathways: MAPK and NF-κB. They also showed that TCD4+ cells cultured in the presence of anti-RAGE antibodies presented a decreased proliferation.52 Other studies using RAGE-/- mice murine models have shown that the presence of RAGE on T cells is necessary for T cell priming and activation by CD28.53,54 Furthermore, RAGE is involved in Th cell differentiation, since RAGE-deficient T cells released higher amounts of IL-10, IL-4, and IL-5, but lower amounts of interferon (IFN)-γ, which suggested an impaired Th1 differentiation and an increased Th2 differentiation in response to TCR activation.53,54 The impact of RAGE on TCD4+ cell differentiation seems to be dependent on polarisation conditions. Indeed, in an allergic asthma murine model, the deficiency of RAGE reduces allergic airway inflammation and inhibits Th2 cell response i.e. Th2 cytokines IgE production and eosinophil recruitment.55,56
Dietary Advanced Glycation End-Products and Allergy
The Maillard reaction generated by heat treatment of food is responsible for conformational changes of proteins and consequently of allergens. Allergens altered by the Maillard reaction are often efficiently uptaken and presented by dendritic cells leading to better CD4+ T cell priming and differentiation toward the Th2 profile. This is associated with an increased proliferation of CD4+ T cells, a marked production of IL-2, IL-4, and IL-5 and a decreased production of IFN-γ and might enhance the allergic response.57,58 Depending on the structure of allergens and the modifications induced by the Maillard reaction, a marked or reduced IgE binding to allergen proteins was observed.59
Consequently, there is now evidence that the Maillard reaction enhances the allergenicity of proteins. Nevertheless, the impact of dietary AGEs on the immune system favouring the Th2 profile and consequently on the predisposition to develop an allergy has not been studied so far. Our preliminary results showed that CML, the main dietary AGE present in infant formula, increases differentiation and proliferation of naïve T cells into the Th2 profile (unpublished data). Furthermore, mice exposed to CML during the sensitisation phase had higher levels of antigen-specific IgE and exhibited more severe allergic reactions.60
CONCLUSION
All together, these studies indicate a potential role of dietary AGEs in allergies. This needs further investigation because of the high levels of AGEs, notably CML, in infant formula and the specific vulnerability of the immune system to early environmental changes. Indeed, there is growing evidence that exposures in the early post-natal period can modify gene expression and disease susceptibility and that these dietary changes play a central role in this epigenetic paradigm. While hydrolysed formulas are often used for primary prevention of allergy, clinical studies show contradictory results on the rates of allergic infants from the breast-fed versus formula-fed populations.13 Since CML levels are particularly high in hydrolysed formula, determining the role of dietary AGEs in allergy susceptibility is crucial for atopic babies who are fed with these types of milk and who are at high risk of allergy. If their role is proved, it would require food industries to modify the infant formula process in order to limit the occurrence of AGEs.