Abstract
Heterotopic pregnancy (HP) is the simultaneous occurrence of intrauterine and ectopic pregnancies (EP). The incidence of HPs occurring spontaneously ranges from 1 in 10,000 to 1 in 30,000. However, this incidence is reported to be 1 in 100 pregnancies following artificial reproductive techniques. HP is a potentially life-threatening condition that is frequently misdiagnosed, as most diagnoses for HPs are delayed, and are only made after rupture of the EP. A high index of suspicion is, therefore, required for an accurate and timely diagnosis in order to reduce maternal morbidity and mortality, which currently stands at 1 in 200,000 live births. The most common risk factors include pelvic inflammatory disease, previous EP, assisted reproduction techniques, and ovarian hyperstimulation syndrome.
Transvaginal ultrasound is the gold standard for diagnosis. As detection of an intrauterine pregnancy often leads to the mistaken exclusion of a concomitant EP, a careful transvaginal scanning of the uterus and appendages should be performed in all females of reproductive age with a positive pregnancy test and red flags in anamnesis, and/or with clinical symptoms. Routine transvaginal ultrasound at Day 27 after embryo transfer could facilitate the diagnosis of HP; however, symptoms onset before or after Day 27 are clues to early diagnosis. MRI can be very helpful in diagnosing atypical cases.
Key Points
1. While heterotopic pregnancies occurring spontaneously is 1 in 10,000 to 1 in 30,000, the rate of incidence is 1 in 100 in females following artificial reproductive techniques.2. Currently, transvaginal ultrasound is the gold standard for diagnosis, and scanning the uterus should be performed in all females who have a positive pregnancy test.
3. Heterotopic pregnancies can occur in any female of reproductive age, especially those who have undergone in vitro fertilisation, but are difficult to diagnose because of their rarity.
INTRODUCTION
Infertility is one of the major health concerns faced by individuals globally.1 The 2021 United Nations (UN) World Population Prospects data sheet estimates that the average worldwide total fertility rate is 2.438 births per woman (bpw), a 0.41% decline from 2020.2 This rate is approximately half of what it was in 1950 (4.700 bpw), and more economically developed countries have even lower rates. The fertility rate in Europe is relatively low, with no country above 2.000 bpw, and has declined further in recent years.1 The Americas have also seen widespread declines in fertility, with a regional fertility rate that is currently below replacement level at 1.900 bpw, ranging from 1.700 in North America to 2.200 in Central America.3
According to the American Pregnancy Association, male infertility accounts for 30% of infertility cases. Moreover, since the female literacy rate is increasing, females are more frequently opting to plan a family later in life due to their career prospects, which in turn makes females more dependent on fertility treatments as fertility decreases with age.1
According to a retrospective study conducted by Pi et al.4 on the widespread application of assisted reproductive technologies (ART), the incidence of heterotopic pregnancy (HP) has increased. HP is a rare complication of pregnancy, in which both extrauterine and intrauterine gestation occur simultaneously.5,6 The reported incidence is 0.6–2.5 out of 10,000 pregnancies.5 The presence of an intrauterine pregnancy (IP) does not rule out the presence of a coexisting ectopic pregnancy (EP). Duverney was the first to report the occurrence of an HP in 1708, after finding an IP during the autopsy of a female who had died from a ruptured EP.7 Devoe and Pratt8 reported a heterotopic rate of 1 in 30,000 pregnancies, an estimate based on a theoretical calculation made in 1948. However, the incidence increases to 1–3 in every 100 pregnancies for pregnancies that follow ARTs, such as in vitro fertilisation (IVF) and gamete intra-fallopian transfer, or patients with a history of pelvic inflammatory disease (PID).9
In this article, the authors present a case of HP after 6 weeks and 3 days of gestation, diagnosed by transvaginal ultrasound (TVUS), which was managed with emergent laparoscopy. The IP course was uneventful, with the delivery of a healthy baby girl at term by caesarean section. This case study will serve as an example to increase awareness on HP early diagnosis and prompt management to improve maternal and fetal outcomes.
MATERIALS AND METHODS
A literature search was carried out to evaluate the occurrence of HP after assisted reproduction therapy, and a total of 31 original articles were included in the analysis. The authors investigated the risk factors, clinical presentation, diagnostic evaluation, treatment, and follow-up recommendations for each case. The authors’ case report was produced on an HP that occurred after IVF and embryo transfer (ET) procedure in a patient post-unilateral salpingectomy, as an increased awareness of this rare life-threatening condition among practitioners is essential.
CASE PRESENTATION
A 31-year-old female (gravida: 2; para: 0; abortion: 0) presented at the authors’ fertility clinic with mild pelvic discomfort and a good general condition otherwise. The patient had previously had a left salpingectomy due to an EP in her medical history, but no other medical conditions were reported. She had been trying to conceive for 3 years before she presented to the clinic and was diagnosed with infertility due to male factor. She underwent an intracytoplasmic sperm injection-IVF procedure and ET 6 weeks and 3 days before the presentation. This was an IVF fresh cycle attempt after previously failing an IVF fresh cycle and one frozen ET.
During the current cycle, the patient had two embryos transferred in Day 3(‘cleavage stage’). The human chorionic gonadotropin (hCG) test was done to check for the hormone hCG in her blood, 2 weeks after the ET procedure that resulted present. As per protocol, the patient was scheduled for follow up at the IVF clinic and to have her first ultrasound (US) scan performed at 7 weeks pregnancy. However, the patient presented at the urgent care clinic 4 days earlier than scheduled as she was experiencing mild discomfort in her right lower quadrant. The obstetrician/gynaecologist on duty performed a US scan, and informed the patient that she had a single vital IP, and sent her home. The next day, being in mild but consistent discomfort, the patient presented to the IVF clinic. A TVUS scan revealed two viable embryos with a crown rump length of 4 mm, corresponding to 6 weeks and 1 day of gestation, and detected minimal free abdominal fluid in the pouch of Douglas (Figure 1).
One intrauterine embryo was identified, and a second embryo was implanted in the right fallopian tube. Doppler ultrasonography revealed that both intrauterine and extrauterine fetuses were viable (Figures 2 and 3). The patient was stable and reported mild vaginal bleeding/spotting and right pelvic discomfort, rated 3 out of 10, which had no radiation and was constant and dull. The patient had a history of left salpingectomy 2 years prior to presentation, but no other history of PID, endometriosis, or trauma. She was found to have a haemoglobin level of 11.2 g/dL, haematocrit value of 27%, and a leukocyte count of 16,000/mm3, and her renal and liver function tests and coagulation parameters were within normal limits. The patient was transferred to the operating room urgently and underwent a laparoscopic procedure. The EP was removed, and the IP made it safely to term delivery. The patient delivered her child at 39 weeks of pregnancy via caesarean section.
As HPs are a rare occurrence in the daily experience of general obstetricians gynaecologists, they are often hard to recognise. This case was a near miss that would have had fatal consequences to the intrauterine embryo and the mother if not diagnosed promptly.
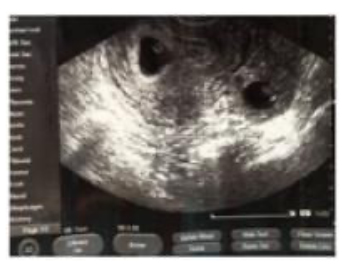
Figure 1: A transvaginal ultrasound displaying the heterotopic pregnancy outlined in this case study.
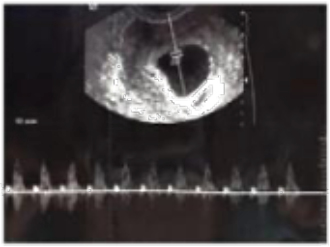
Figure 2: A Doppler ultrasound of a viable extrauterine pregnancy.
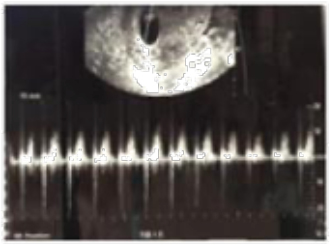
Figure 3: A Doppler ultrasound of a viable intrauterine pregnancy.
DISCUSSION
Risk Factors
HP, defined as the simultaneous occurrence of IPs and EPs, is a potentially life-threatening and frequently misdiagnosed condition.10 The most common risk factors for EP include PID, intrauterine devices, adhesions, a previous history of EP, ARTs, and ovarian hyperstimulation syndrome.11,12 Pi et al.4 also reported that tubal infertility and pelvic adhesion increase the risk of HP among patients undergoing IVF treatment; however, a cohort study conducted by Xiao et al.13 reported no significant difference in the incidence of HP in fresh IVF cycles versus frozen-thawed cycles. In a retrospective study by Lv et al.,14 the authors found that a history of EP and previous tubal surgery may increase the risk of HP. In addition, low levels of serum hCG and oestrogen in the patient on Day 14 after ET could indicate the incidence of HP. Jeon et al.11 reported that patients undergoing IVF and ET with a history of EP, abortion, and ovarian hyperstimulation syndrome may be at increased risk for HPs compared with the control group of other IVF patients. Liu et al.15 indicated that the transfer of two or more embryos in an IVF procedure, low β-hCG and progesterone levels on Day 14 after ET, and vaginal bleeding should be considered as predictors for HPs.
A retrospective study conducted by Luo et al.16 on 1,476 pregnancies following IVF-ET procedures reported 12 (0.81%) such HP cases. In this paper, they attributed the increase in HP cases to the increased incidence of genital infections and widespread use of ovulation induction agents.16 Exogenous hormones, uterine contractions that occur during the transfer of the fertilised embryo, inadvertent introduction of the embryo directly into the fallopian tube, and the volume and number of embryos transferred are all risk factors that are specifically encountered in ART.16,17 Risk factors for HPs, as noted by Nabi et al.,10 also include a personal history of tubal diseases and the use of ART. Furthermore, Soares et al.18 observed that the risk factors for an HP closely mirror those of an EP, and, based on a meta-analysis performed by Ankum et al.,19 these were found to be: a history of EP, tubal surgery, or pathology; in utero diethylstilbestrol exposure; and previous genital infections, including PID, chlamydia, and gonorrhoea.
Gergolet et al.20 described a case of HP that occurred after a single embryo and blastocyst transfer. In this example, intercourse took place on the day of hCG administration to a patient, where an oocyte was not collected during the oocyte retrieval procedure, which resulted in an HP in Week 11 of pregnancy.
As with EPs, the most common ectopic site for HPs is the fallopian tube; implantation in the cervix, ovary, abdomen, and pelvis is extremely rare.10 Approximately 60–70% of HPs result in live births, with similar outcomes to singleton pregnancies.10 Luo et al.16 reported this figure to be 66.7% at the Reproductive Centre in Guangdong Women and Children’s Hospital, China. The high mortality rate of intrauterine gestation may be attributed to delays in diagnosis, but, if left to progress, this condition could also lead to maternal mortality.10
Clinical Presentation
In a study conducted by Reece et al.21 that reviewed 589 cases of HPs globally, abdominal pain was reported to be the most common presenting symptom. Other signs and symptoms most commonly found in support of a presumptive diagnosis of an HP included the presence of an adnexal mass, peritoneal irritation, and an enlarged uterus.21 However, these signs and symptoms are unspecific, and may be found in other gynaecological and non-gynaecological conditions such as a miscarriage, EP, IP with adnexal torsion, appendicitis, a bowel obstruction, cholecystitis, or pancreatitis.18, However, it is rare for an HP to present with vaginal bleeding.21 Nabi et al.10 reported the non-specific presence of abdominal pain in 72% of HPs and vaginal bleed in 54% of HPs. It was reported by Soares et al.18 that, as a consequence, most diagnoses for HPs are delayed, and are only made after rupture of the EP. A high index of suspicion is, therefore, required for an accurate and timely diagnosis in order to reduce maternal morbidity and mortality, which currently stands at 1 in 200,000 live births.22 To complicate matters further, up to 24% of cases may present asymptomatically.10 In a retrospective review by Lyu et al.23 on 55 patients with HPs, it was found that only 29.1% of patients presented with abdominal pain, while 34.5% of patients were asymptomatic prior to diagnosis.
Diagnostic Techniques
A review by Talbot et al.,24 which looked at cases published prior to 2010, noted the increased role of US scans in the definitive diagnosis of HPs. Indeed, TVUS remains the gold standard for the diagnosis of an HP.18 However, as the detection of an IP often leads to the mistaken exclusion of a concomitant EP, it is recommended that careful adnexal US assessment be carried out to mitigate the risk of missing an HP.18 In the study by Lyu et al.,23 the authors suggested that routine TVUS at Day 27 following ET could facilitate the diagnosis of an HP, leading to fewer missed diagnoses. Current National Institute for Health and Care Excellence (NICE) guidelines also outline the importance of scanning the uterus and both adnexae when performing a TVUS early in the pregnancy to check for an HP.22
Separately, studies by Yoshigi et al.25 and Si et al.26 that aimed to determine the role of MRI in the early diagnosis of an EP reported that the use of T2-weighted imaging was highly accurate in the detection and diagnosis of EPs. Masselli et al.27 also retrospectively reviewed 150 patients with a suspected diagnosis of EP, and analysed the clinical, US, and MRI features of 15 unusual cases. The authors reported that diffusion- and fat saturation T1-weighted images were the most accurate for the detection of EPs, picking up 100.0% and 93.3% of the 15 cases, respectively.27 As such, the authors concluded that the use of MRI for the early diagnosis of early EPs should be considered, following negative US findings in some highly suspicious cases.27
Management
Treatment methods for EPs currently include medical management with methotrexate, surgical management with salpingostomy or salpingectomy, and expectant management.28 It appears that the surgical treatment of HPs does not appear to affect the rates of first trimester fetal loss or live birth.12 However, the use of methotrexate in HPs should be avoided, given its teratogenic effects on the viable IP.12,29 Li et al.30 performed a prospective study on 64 patients diagnosed with HPs between January 2003 and June 2014, of which 12 patients were excluded on grounds of a non-viable IP. The remaining 52 patients either received expectant management (n=20), surgical management (n=27), or transabdominal US-guided transvaginal aspiration of the ectopic gestational embryo (n=5).30 The surgical management group included those who underwent an emergency and elective laparotomy and laparoscopy.30 Of the 20 patients managed expectantly, six eventually converted to surgical treatment. Of the 27 patients managed surgically, there were four abortions and one stillbirth. Transabdominal US-guided transvaginal aspiration resulted in 100% live births without the need for secondary conversion to any other treatment methods.30 This led the authors to conclude that transabdominal US-guided transvaginal aspiration gave rise to the lowest abortion rate and best maternal outcome, while surgical management led to the highest abortion rates and expectant management resulted in the worst maternal outcomes.30 However, the degree of difficulty in utilising transabdominal US-guided transvaginal aspiration of the ectopic embryo depends on the location of the ectopic gestational sac, and should be attempted only when this can be clearly visualised.30
Yu et al.31 reported that surgery is still the most frequently chosen form of treatment for the management of an HP, and that it involves a salpingectomy in most cases, although the selected method ultimately depends on the actual clinical condition of the patient. It is further recommended by Ciebiera et al.32 that the manipulation of the uterus be kept to a minimum during surgery in order to protect the IP from potential complications.
Recommendations
The patient study outlined in this article is a classic case, presenting with the typical risk factors associated with a left salpingectomy and the use of ART, as well as the most commonly encountered complaint of mild abdominal discomfort. Despite this, the general obstetrician/gynaecologist attending physician was unable to arrive at an accurate diagnosis. Given the difficulty encountered in diagnosing HPs, particularly in patients who do not present classically or in whom physicians do not have a high degree of suspicion for, and that ART and the incidence of HPs have been on the rise in recent years, it appears advisable to recommend that physicians perform a TVUS on ART patients at 27 days following the ET process, as reported by Lyu et al.23 Ultimately, the patient underwent an emergency laparoscopy to remove the ectopic gestational embryo, allowing for the successful carriage of the IP to term.
From this case, we can see that it is indeed challenging to diagnose an HP in view of the non-specific clinical and laboratory findings surrounding the condition. It is, however, essential to highlight the importance of correct diagnosis and prompt treatment, as failure to do so poses a threat to both the viable and the developing fetus, as well as to the mother. Every red flag should, therefore, be carefully evaluated and consulted by a fertility team, if required.33
CONCLUSION
HPs are very difficult to diagnose because of the rarity of their occurrence in everyday practice. An HP may occur in any woman of reproductive age, especially in induced ovulation or IVF cases. In patients undergoing IVF treatment, additional attention is recommended during the first US scan, especially in females where more than one embryo is transferred. Careful scanning of the uterus and appendages is necessary in all females of reproductive age with clinical symptoms. Routine TVUS could facilitate the diagnosis of HP, and timely diagnosis can change the outcome of the pregnancy and decrease complications for the patient. Laparoscopic intervention is the treatment of choice in haemodynamically stable patients, as a laparoscopy can safely achieve the removal of ectopic implantation with minimal trauma whilst avoiding intraperitoneal haemorrhage and complications with the concomitant IP. A laparotomy has more associated complications, but these are indicated by severe intra-abdominal bleeding or haemorrhagic shock.








