Chairperson: Nikos Papadopoulos1,2
Speakers: Jan Knol,3,4 Kirsten Beyer,⁵ Anna Nowak-Wegrzyn6,7
1. University of Manchester, Manchester, UK
2. University of Athens, Athens, Greece
3. Danone Nutricia Research, Utrecht, the Netherlands
4. Wageningen University, Wageningen, the Netherlands
5. Charité – Universitätsmedizin Berlin, Berlin, Germany
6. Hassenfeld Children’s Hospital, New York City, New York, USA
7. New York University, New York City, New York, USA
Disclosure: Prof Papadopoulos has received personal fees from Novartis, Nutricia, HAL Allergy Group, Menarini/Faes Farma, Sanofi, Mylan/Meda, biomay, AstraZeneca, GlaxoSmithKline, MSD, ASIT Biotech, Boehringer Ingelheim; and grants from Gerolymatos International SA and Capricare. Prof Knol is a full-time Director of the Gut Biology & Microbiology Platform of Danone Nutricia Research, the Netherlands. Prof Beyer has received research grants from Aimmune Therapeutics, ALK, Stiftung Berliner Sparkassen, Danone Nutricia, DBV Technologies, DST Diagnostic, GoodMills, HiPP, Hycor Biomedical, InfectoPharm, Thermo Fisher Scientific, VDI; has received other research support from the European Union (EU), German Research Foundation (DFG), Federal Ministry of Education and Research (BMBF), Federal Ministry of Food and Agriculture (BMEL); has been on the speakers bureau or received honoraria from Aimmune Therapeutics, Allergopharma, Bencard Allergie, Danone Nutrica, DiText, Hammer & Rall Media, InfectoPharm, Mylan/Meda, Nestle, Thermo Fisher Scientific; and has been a consultant for or a member of the advisory board for Aimmune Therapuetics, ALK, Bausch + Lomb, Bencard Allergie, Danone Nutrica, DBV Technologies, HiPP, Hycor Biomedical, InfectoPharm, Mabylon, Mylan/Meda, Nestle, Novartis. Prof Nowak-Wegrzyn has received honorarium for this presentation from Nutricia; and has received a research grant and has been a site principal investigator for the clinical study (PRESTO) of amino acid-based formula with synbiotics.
Acknowledgements: Medical writing assistance was provided by Jennifer Taylor, London, UK.
Support: The publication of this article was funded by Nutricia Specialized Nutrition.
Citation: EMJ. 2020;5[4]:20-28.
Meeting Summary
This symposium took place during the virtual Food Allergy and Anaphylaxis Meeting and European Consortium on Application of Flow Cytometry in Allergy (FAAM–EUROBAT) Digital 2020, hosted by the European Academy of Allergy and Clinical Immunology (EAACI). Focussing on the use of synbiotics to manage infants with cow’s milk allergy (CMA), the speakers discussed the importance of the gut microbiota, and the impact of synbiotics on CMA outgrowth and reported infection outcomes. Prof Knol set the scene by describing how infants with CMA often have an altered gut microbiota compared to healthy breastfed infants. He explained the composition of synbiotics and presented data from three randomised controlled trials (RCT) that have demonstrated how synbiotics rebalanced the gut microbiota in infants with CMA, bringing them closer to that of healthy breastfed infants. Prof Beyer discussed cow’s milk as a common food allergen, and how children often develop tolerance naturally. She then presented results from the PRESTO trial, which showed that approximately one-half of children with IgE-mediated CMA who received an amino acid-based formula (AAF), with or without synbiotics, developed cow’s milk tolerance within 12 months. The results were in line with what has been reported in the literature for this population. Prof Nowak-Wegrzyn highlighted how simple infections can have a detrimental effect on young children and lead to treatment with antibiotics. Infants and children with CMA are more susceptible and prone to recurrent infections. Three RCT have suggested that an AAF with synbiotics can reduce infections and the use of antibiotics in children with IgE-mediated CMA and non-IgE-mediated CMA. The meeting concluded with a panel discussion.
Synbiotics: Inspiration from Human Milk and the Effect on the Gut Microbiota
Professor Jan Knol
Early life is a critical time for the development of the gut microbiota and maturation of the immune system. The infant gut is almost sterile at birth and needs to be colonised to form the gut microbiome. The immune system is also naïve at birth and needs to learn to distinguish between good and dangerous signals from the environment. These developmental processes go hand in hand.
The gut hosts 70-80% of the human body’s immune cells,1 and its microbiota support immune function and development.2-5 There is also crosstalk between the gut microbiota and the immune system.2,3 The gastrointestinal (GI) mucosal immune system plays a pivotal role in the maintenance of immune homeostasis and is crucial for suppressing responses to harmless antigens and beneficial bacteria, as well as responding to threats such as toxins or pathogenic bacteria.
The gut microbiota acts as a barrier against pathogens. Imbalances of the gut microbiota, known as dysbiosis, can trigger several immune disorders through the activity of T cells, such as allergic reactions and infections.6,7 In contrast, a healthy, balanced gut microbiota acts as a barrier against the infiltration and colonisation of pathogens, thereby protecting infants against infections at the epithelial layer.8-10 A number of mechanisms are at play in the healthy gut to prevent the growth of pathogens. Beneficial bacteria compete with pathogens for adhesion sites and nutrients in order to produce antimicrobial peptides and bacterial metabolites such as short chain fatty acids (SCFA). The acidic environment hinders pathogen growth and enables healthy bacteria, such as bifidobacteria and lactobacilli, to thrive. Healthy bacteria also support the integrity of the epithelial and mucosal barrier with tight junctions and production of mucus.
Multiple factors can impact the gut microbiota in early life and potentially cause dysbiosis.11 These include the maternal microbiota and duration of gestation, with preterm infants having an aberrant microbiome development. Mode of delivery also plays a role, as infants born via caesarean section have a different exposure to microbes compared to those delivered vaginally. Other factors include early dietary feeding (breast milk versus formula), use of antibiotics and/or probiotics, and environmental factors such as family size and exposure to pets.
Infants with CMA often have an altered gut microbiota compared to healthy breastfed infants.12 Healthy infants have dominant levels of Bifidobacterium species, Faecalibacterium prausnitzii, and Lactobacillus species. In infants with allergies, these numbers are lower and there is a greater prevalence of adult-like, potentially pathogenic bacteria, such as Enterococcus faecalis, Clostridium difficile, and Campylobacter.
Breastfeeding is the preferred nutrition for infants and is undisputedly the best nourishment for all infants worldwide. It also contains many components, such as live bacteria, prebiotic oligosaccharides, and lactose, which all stimulate the growth of beneficial bacteria and the healthy development of the immune system. When breastfeeding is not possible, hypoallergenic formulas are recommended to manage infants with CMA. However, traditional hypoallergenic formulas, including extensively hydrolysed formula and AAF, lack the microbiota-stimulating factors of breast milk.
This is where the synbiotic concept comes into play, providing the best of both worlds. Synbiotics are a combination of prebiotics (the substrates for growth of beneficial bacteria) plus probiotics (live bacteria that, when administrated in adequate amounts, confer a health benefit).13-16 This blend of pre- and probiotics mimics the composition of human breast milk and the two ingredients work synergistically to target gut microbiota dysbiosis.17
Human breast milk contains thousands of different oligosaccharides,18 162 of which have been identified.19 These oligosaccharides have multiple functions but of most importance is the ability to act as a substrate for the growth of bacteria in babies’ GI tract, i.e., prebiotics. The benefits of prebiotics have been acknowledged in the World Allergy Organization (WAO)-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P).20 Multiple studies have been conducted on the specific prebiotic mixtures’ short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides (scGOS/lcFOS) and short-chain fructo-oligosaccharides and lcFOS (scFOS/lcFOS) in 9:1 ratios. These blends mimic the diversity, quantity, and functionality of human milk oligosaccharides in human breast milk.21-24
A synbiotic is created by taking the prebiotic and adding the probiotic Bifidobacterium breve M-16V® (Morinaga Milk Industry, Tokyo, Japan), a unique infant strain. B. breve is one of the most commonly isolated Bifidobacterium species from human breast milk.2,25 It is a natural species in the infant gut and is one of the predominant Bifidobacterium species in breastfed infants.26,27 The M-16V strain was selected for its ability to reduce allergic responses, as shown in preclinical28,29 and clinical studies,30,31 and for its proven safety.32 For example, M-16V induces no undesired antibiotic resistances and is free from major allergens (cow’s milk, eggs, wheat, nuts, peanut, soya, and fish/shellfish protein).
Six RCT on either extensively hydrolysed formula or AAF including synbiotics have now been published, demonstrating the safety and effectiveness in healthy, high-risk, and allergic infants. An extensive clinical trial programme has been conducted on an AAF with synbiotics (B. breve M-16V with scFOS/lcFOS) over more than 10 years, of which three specifically studied infants with CMA. A study by Harvey et al.33 showed that the formula was safe, well tolerated, promoted normal growth in healthy infants, and was hypoallergenic according to the American Academy of Pediatrics (AAP) guidelines. A study by Burks et al.34 demonstrated that an AAF with synbiotics was safe and well tolerated in infants with IgE-mediated CMA and non-IgE-mediated CMA. Meanwhile, a study by Candy et al.35 in infants with non-IgE-mediated CMA showed that synbiotics were able to balance the gut microbiota, bringing it closer to the gut microbiota seen in breastfed infants after 8 weeks. The ongoing PRESTO trial is investigating oral tolerance to cow’s milk and the incidence of future allergies in infants with IgE-mediated CMA.
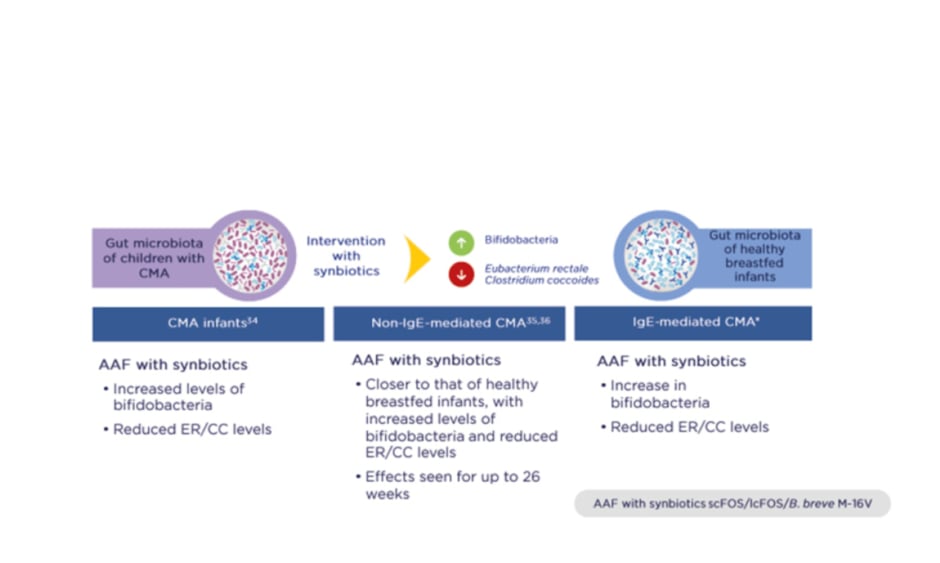
Importantly, three RCT have shown that synbiotics rebalance the gut microbiota in infants with CMA so that it more closely resembles that of healthy breastfed infants (PRESTO, unpublished data; Figure 1).34-36 Intervention with synbiotics increased beneficial bifidobacteria while reducing levels of bacteria more commonly seen in adults, namely members of the Eubacterium rectale/Clostridium coccoides genera. The gut environment also shifted to a healthier state, with increased levels of SCFA and a lower pH.37
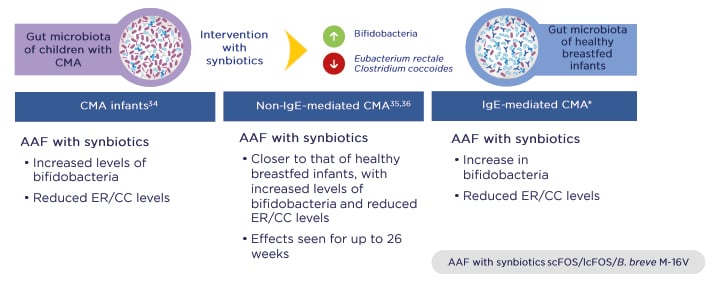
Figure 1: Three randomised controlled trials demonstrated that synbiotics rebalance the gut microbiota in infants with cow’s milk allergy and bring it closer to that of healthy breastfed infants.
*PRESTO, unpublished data.
AAF: amino acid-based formula; B. breve M-16V: Bifidobacterium breve M-16V; CC: Clostridium coccoides; CMA: cow’s milk allergy; ER: Eubacterium rectale; lcFOS: long-chain fructo-oligosaccharides; scFOS: short-chain fructo-oligosaccharides.
Adapted from Burks et al.,34 Candy et al.,35 and Fox et al.36
In the panel discussion, Prof Papadopoulos asked how synbiotics compare to human milk oligosaccharides, and whether synbiotics are safe. Prof Knol answered that the evidence on synthetic human milk oligosaccharides in infant formula is still scarce but he highlighted that synbiotics are a different concept compared with synthetic human milk oligosaccharides because they also contain live bacteria on top of the substrates these bacteria need. In this way, synbiotics bring live microbes into the ecosystem and feed them, leading to SCFA production and growth of bifidobacteria. This whole system approach supports the developing microbiome in early life so that it is similar to that of breastfed infants. The specific synbiotic mixture that has been well studied has proven safety in healthy infants, allergic infants, and in preterm infants.
In conclusion, a healthy gut microbiota is important for the development of the immune system and defence against infections. Human breast milk helps this process because it contains antimicrobials/antibodies, as well as substrates for beneficial bacteria, which steer healthy maturation. Synbiotics mimic human breast milk by combining prebiotics and probiotics to target the gut microbiota dysbiosis seen in infants with CMA. Three RCT have confirmed that a specific synbiotic mixture is able to rebalance the gut microbiota in infants with CMA, bringing it closer in composition, and also in activity, to that of healthy breastfed infants.
Effect of Synbiotics on Tolerance Development in Infants with IgE-Mediated Cow’s Milk Allergy
Professor Kirsten Beyer
Cow’s milk is one of the most common food allergens in early life. The prevalence of IgE-mediated CMA ranges from 0.5% to 3.0% at 12 months of age.38-40 The most common symptoms are immediate type reactions, which are usually IgE-mediated, followed by worsening of atopic eczema, and non-IgE-mediated GI diseases.
In the first 3 years of life, cow’s milk is a typical trigger of food-induced anaphylaxis, as shown in the European Anaphylaxis Registry of 1,970 children.41 Natural tolerance development is common. Data from the EuroPrevall birth cohort of >12,000 children from nine European countries followed-up over 3 years showed that approximately 70% of children with CMA outgrew their allergy within 1 year.40 These data confirm previous results from another cohort study conducted 30 years ago.42 However, other studies generate a heterogeneous picture, with slower outgrowth of CMA.43,44 This mixed picture may be explained by different study populations and methodologies; for example, by retrospective collection of data or lack of follow-up at fixed intervals with oral food challenges. Infants with IgE-mediated CMA usually experience later outgrowth compared with those with non-IgE-mediated CMA; this was shown in three studies conducted in children with each type of CMA.40,42,45
The ongoing PRESTO trial has been investigating the effect of an AAF with synbiotics on the natural history of CMA. Conducted in 18 study sites across six countries, the trial was designed to evaluate the development of cow’s milk tolerance and safety of an AAF including synbiotics in infants with IgE-mediated CMA. The trial enrolled 169 infants aged 0-13 months with IgE-mediated CMA confirmed through an oral food challenge or anaphylactic history. Infants with cow’s milk protein-induced anaphylaxis or multiple food allergies were also included.
Participants were randomly allocated to an AAF with synbiotics (scFOS/lcFOS/B. breve M-16V; n=89) or an AAF without synbiotics (n=80) for 12 months. After the 12-month intervention, the children were followed-up for a further 24 months for a total of 36 months. Follow-up will continue for another 36 months (i.e., a total of 6 years). The primary outcome was the proportion of subjects developing tolerance to cow’s milk after 12 months of intervention, as measured by a double-blind, placebo-controlled food challenge. Secondary outcomes of the trial will be development of tolerance to cow’s milk at 24 and 36 months. Other endpoints include stool outcomes, adverse events, concomitant medication use, and growth.
Results have been reported after 12-month intervention and a further 12 months of follow-up (i.e., a total of 24 months).46 Looking at the study population overall, after 12 months of intervention, 49% of the children had outgrown their CMA (Figure 2). At the 24-month follow-up, 62% had outgrown the disease.46
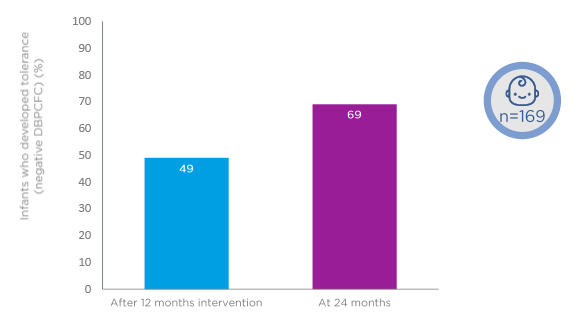
Figure 2: Approximately 50% of children receiving amino acid formula with or without synbiotics develop cow’s milk tolerance after 12-month intervention.
DBPCFC: double-blind, placebo-controlled food challenge.
Adapted from Chatchatee et al.46
Outgrowth was good in both study groups at 12 and 24 months, with no significant differences in the proportions developing tolerance. After 12 months of the intervention, 45% of the group allocated to an AAF with synbiotics developed cow’s milk tolerance compared with 52% of the group receiving an AAF without synbiotics (p=0.401). At 24 months, 64% of the test group had developed cow’s milk tolerance compared with 59% of the control group (p=0.530).46
Outgrowth in the PRESTO study population was in line with three studies that previously reported on the proportion of infants with IgE-mediated CMA who achieved natural tolerance to cow’s milk after 12-18 months, which ranged from 38% to 57%.40,42,46 An additional study by Canani et al.47 examined an extensively hydrolysed casein formula (eHCF), with or without the probiotic Lactobacillus rhamnosus GG. Prof Beyer said that looking at these results against those of PRESTO gives the impression that children in PRESTO developed cow’s milk tolerance faster than those receiving an eHCF with or without L. rhamnosus GG.
Regarding safety, during the 12-month PRESTO intervention, significantly fewer infants in the test group (8.8%) required hospitalisation as a result of serious adverse events categorised as infections, compared with the control group (20.2%; p=0.036) (Figure 3).46
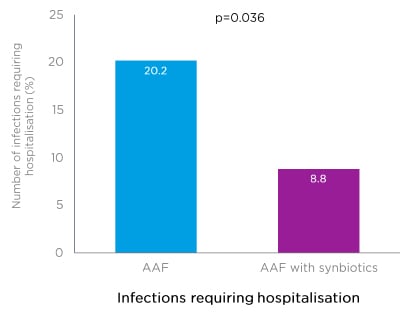
Figure 3: An amino acid-based formula with synbiotics led to reduced infections requiring hospitalisation in the PRESTO trial.
AAF: amino acid-based formula.
Adapted from Chatchatee et al.46
During the panel discussion, a query was raised about the possible reasons why outgrowth of CMA in the PRESTO trial was similar in infants who received synbiotics compared with those who did not. Prof Beyer said that the researchers were very happy to see the fantastic tolerance development, which was similar to observations in other studies, particularly because previous experts have questioned whether an AAF may not induce tolerance. Contrary to that view, the AAF used in PRESTO gave similar, and possibly faster, tolerance development to an eHCF in another study.47 Further, Prof Beyer said the investigators were slightly disappointed that there was no difference between the two groups, and the result remains unexplained. She is very curious to see the long-term effects on other food allergies and other atopic diseases from this trial.
Prof Papadopoulos queried whether the development of cow’s milk tolerance in 70% of children is perhaps the maximum that can be achieved. He also noted that children in PRESTO might have been exposed to some milk allergen, which could have influenced the results. Regarding the possibility of a threshold of tolerance development, Prof Beyer said this was unlikely and was hopeful that the remaining 30% of children would outgrow their allergy. She added that there is still the potential for a difference between study groups at the 6-year follow-up. As for exposure to cow’s milk, Prof Beyer highlighted that children with CMA often receive small amounts as a result of contamination in bakery products and other foods, so this was unlikely to have had an impact on the results of the trial.
Prof Beyer was asked whether her views on how to manage CMA have changed with the results of PRESTO. She replied that the recommendation should still be to eliminate cow’s milk from the diet. Following the PRESTO trial, the replacement formula could be an AAF, given that it demonstrated similar tolerance compared to an eHCF.
In conclusion, in the PRESTO trial, approximately one-half of children with IgE-mediated CMA developed oral tolerance to cow’s milk within 12 months, and an additional 13% outgrew their allergy within 24 months. The tolerance development observed in PRESTO is in line with the literature. In addition, children in PRESTO who received an AAF with synbiotics required fewer hospitalisations as a result of infection compared with those allocated to an AAF without synbiotics.46
Clinical Benefits of Synbiotics Beyond the Dietary Management of Infants with Cow’s Milk Allergy
Professor Anna Nowak-Wegrzyn
Simple infections can have a considerable impact on the lives of young children. During the first 3 years of life, children experience multiple infections, particularly of the upper respiratory tract.48,49 While those infections are rarely a cause of mortality in well-developed industrialised countries, they do have a detrimental impact on overall childhood health, hospitalisation rates, and quality of life. In addition, the increased use of healthcare resources, parental work absenteeism, and secondary infections in parents and siblings creates an economic burden for society.50,51
Infants and young children are predisposed to infections because of the immaturity of the immune system at birth. Maturation occurs during the first 3-5 years of life. The Copenhagen Prospective Study on Asthma in Childhood 2000 (COPSAC 2000) reported a median of 14 infectious episodes in otherwise healthy children aged 0-3 years, with a large and unexplained variation in individual susceptibility (the number of infections ranged from 2 to 43).50 Respiratory tract infections were the most common, with fewer cases of fever and GI infections. Infections peaked at around 1 year of age and declined thereafter. The study also found that 25% of infections were treated with antibiotics, with higher rates of use for ear infections and respiratory tract infections. The most frequently used drug was amoxicillin (59.4%), followed by penicillin (27.9%).
Infants and young children with CMA are more susceptible and more prone to recurrent otitis media (ear infection) compared to those without CMA.52,53 A retrospective analysis of 280 infants with CMA showed that sensitisation to whey protein was associated with a 4-fold increased risk of recurrent respiratory tract infection before 2 years of age.54
As discussed by Prof Beyer, the PRESTO trial found that infants with IgE-mediated CMA who received an AAF with synbiotics had significantly fewer hospitalisations as a result of infection compared with those allocated to an AAF without synbiotics (8.8% versus 20.2%; p=0.036) (Figure 3).46 This finding was consistent with previously reported data from two RCT of AAF with synbiotics (a blend of scFOS/lcFOS and B. breve M-16V).
The first trial, in infants with CMA, reported that the synbiotic formula led to significantly fewer infections (2% versus 18%; p=0.008) and use of systemic antibiotics (17% versus 34%; p=0.049), specifically amoxicillin (9% versus 32%; p=0.004), compared with an AAF without synbiotics.34 The second trial, which enrolled children with non-IgE-mediated CMA, found significantly fewer ear infections (0% versus 20%; p=0.011) and less use of anti-infectives (8.6% versus 34.4%; p=0.018) in infants fed with the synbiotic formula.35,36 A preliminary systematic review of the three trials suggested that the synbiotic-containing AAF had a protective effect against ear infections in children with CMA and was linked with reduced antibiotic use for ear infections compared with an AAF without synbiotics.55 This effect is of special relevance for atopic infants who are born with a predisposition to develop frequent infections as a result of the immaturity of their T helper Type 1 anti-infective responses. In summary, new generation hypoallergenic infant formulas with synbiotics mimic the immunomodulatory effects of breast milk, improve the profile of the gut microbiota, and result in fewer infections in early life.
During the panel discussion, Prof Nowak-Wegrzyn was asked to explain the differences in infection rates between breastfed and formula-fed infants and the benefits observed with synbiotics. She highlighted that breastfed infants generally have fewer upper respiratory and GI infections compared with those fed with formulas. Children with CMA are more prone to infections than healthy infants, which often leads to prescription of oral antibiotics, even for viral infections. This unnecessary use of antibiotics contributes to disturbed gut microbiota. However, there could be a remedy: an exploratory analysis in >300 infants with CMA from three trials showed the same signal, namely that an AAF with synbiotics may reduce ear infections and associated antibiotic usage compared to an AAF without synbiotics (ear infections: 0-6% with synbiotics versus 11-20% without synbiotics; medication for ear infections: 0-4% with synbiotics; 9-17% without synbiotics).55
Prof Nowak-Wegrzyn was asked whether she would advise parents to use synbiotics, and in which situations and at what time. She replied that very strong evidence is needed to publish a guideline, and she thinks that the point will be reached when there is sufficient data to recommend the use of synbiotics. Breastmilk continues to be the best source of nutrition for all infants, including those with CMA, and should be promoted as much as possible. If formula is indicated, she would consider recommending one with synbiotics because growth and safety are equivalent to formulas without synbiotics and the rate of infections requiring hospitalisation appears to be lower. She predicted that in future, synbiotics will be indicated in situations in which the gut microbiota may be disturbed, such as infants delivered by caesarean section, or when the mother is receiving antibiotics during pregnancy or lactation.