Interviewees: Tjalf Ziemssen,1,2 Diego Centonze3,4
1. Centre for Clinical Neuroscience, Carl Gustav Carus University Hospital, Dresden, Germany
2. Dresden University of Technology, Dresden, Germany
3. Tor Vergata University of Rome, Rome, Italy
4. IRCCS Neuromed Mediterranean Neurological Institute, Pozzilli, Italy
Disclosure: Prof Ziemssen has received grants and personal fees from Almirall, Bayer, Biogen, Celgene (a Bristol Myers Squibb company), Merck, Novartis, Sanofi, and Teva. Prof Centonze is an Advisory Board member for Alexion, Almirall, Bristol Myers Squibb, Bayer Schering, Biogen, Celgene (a Bristol Myers Squibb company), GW Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi-Genzyme, and Teva; has received honoraria for speaking or consultation fees from Almirall, Bayer Schering, Biogen, Bristol Myers Squibb, GW Pharmaceuticals, Lundbeck, Merck Serono, Novartis, Roche, Sanofi-Genzyme, and Teva; is a principal investigator in clinical trials for AbbVie, Bayer Schering, Biogen, Merck Serono, Mitsubishi, Novartis, Roche, Sanofi-Genzyme, and Zambon; and his preclinical and clinical research has been supported by grants from Bayer Schering, Biogen, Celgene (a Bristol Myers Squibb company), Lundbeck, Merck Serono, Novartis, Roche, Sanofi-Genzyme, and Teva.
Acknowledgements: Writing assistance was provided by Samantha Stanbury, Stockport, UK.
Support: The publication of this article was funded by Celgene, a Bristol Myers Squibb company. The key opinion leaders interviewed did not receive reimbursement for their time or their input to the article.
Citation: EMJ Neurol. 2020;8[Suppl 2]:2-8.
Interview Summary
This article is based on interviews conducted in September 2020 with two leading experts in neurology, Prof Ziemssen from Germany and Prof Centonze from Italy, who discussed the evolving role of sphingosine 1-phosphate (S1P) receptor modulators for multiple sclerosis (MS). Expansion of the S1P receptor modulator class, with the recent approval of two second-generation S1P receptor modulators, ozanimod and siponimod, warrants consideration of what this means for the role of S1P receptor modulators in the management of patients with MS.
The first S1P receptor modulator, fingolimod (Gilenya® [Novartis, Basel, Switzerland]), was approved by the European Medicines Agency (EMA) in March 2011. Fingolimod is indicated, in the European Union (EU), for the treatment of highly active relapsing-remitting MS (RRMS), including in patients whose disease remains highly active despite adequate treatment with at least one disease-modifying therapy (DMT), or those with rapidly-evolving severe disease.1 Siponimod (Mayzent® [Novartis]) was approved by the EMA in January 2020 for the treatment of secondary progressive MS2 and ozanimod (Zeposia®, Bristol Myers Squibb, New York City, New York, USA) received EMA approval in May 2020 for the treatment of RRMS in patients with active disease, as defined by clinical or imaging features.3
The approval of these new compounds marks a potential shift in the treatment landscape, expanding the range of patients with MS for whom S1P receptor modulators may be a suitable treatment option. In this article, Prof Ziemssen and Prof Centonze provided their perspectives on this topic, primarily focussing on the role of S1P receptor modulators in RRMS, including their potential use early in the disease course.
LEADING EXPERT INSIGHTS: CLINICAL EXPERIENCE WITH S1P RECEPTOR MODULATORS
Neurologists are familiar with S1P receptor modulators as a class, largely based on several years’ experience with fingolimod. Siponimod and ozanimod became available in 2020, which provided the opportunity for some experience of using next-generation S1P receptor modulators in clinical practice. Although, as Prof Ziemssen acknowledged, the introduction of new treatments in MS centres had not been optimal, owing to the challenges of managing MS during the coronavirus disease (COVID-19) pandemic. S1P receptor modulation has become the backbone of MS treatment in German specialist MS centres, and Prof Ziemssen indicated that nonspecialist neurologists are also familiar with fingolimod, with most MS patients in Germany being managed in general neurology centres. When fingolimod was first introduced, it was only used in patients with the most severe and highly active disease, but in recent years it has become clear that high-efficacy DMT, such as S1P receptor modulators, can be used earlier in the disease course, when indicated. Ozanimod can be used for first- or later-line treatment based on its indication for RRMS with active disease.
Prof Centonze described fingolimod as a ‘1.5-line’ treatment in Italy, primarily indicated for and often used as second-line treatment, in patients whose disease remains active on first-line treatment. However, benefits of early prescribing of high-efficacy drugs are recognised, so it is not uncommon to use fingolimod as a first-line treatment in clinical practice. It is not reserved only for patients switching for the second or third time; other potent DMT such as natalizumab (Tysabri® [Biogen, Cambridge, Massachusetts, USA]) or ocrelizumab (Ocrevus® [Genentech, South San Francisco, California, USA]) tend to be used in these patients. Therefore, both in Italy and Germany, there is a precedent for the use of S1P receptor modulators in patients with relatively early MS.
ESTABLISHED CLASS SAFETY PROFILE
Perceptions of the safety of S1P receptor modulators as a class have been largely influenced by fingolimod, which has a well-established, long-term safety profile based on several years’ of clinical experience. Prof Centonze felt that it is broadly perceived as a ‘safe’ drug, supported by extensive published literature. The second-generation S1P receptor modulators appear to have improved safety and tolerability profiles, in Prof Ziemssen’s view of the clinical trial data, although class side effects including cardiac events and macular oedema are still observed to some extent. However, he indicated that these are unlikely to be of concern to neurologists, who understand potential class safety concerns with fingolimod and have adapted to implementing safety measures such as ECG and heart rate monitoring upon initiation of treatment. Dosing schedules for ozanimod and siponimod recommend gradual dose-escalation during the first few days of treatment to reduce the risk of bradycardia and to mitigate the need for cardiac monitoring in many patients. For those in whom monitoring is required (e.g., patients with relevant pre-existing cardiac risk factors), Prof Ziemssen did not see it as a major problem because neurologists are accustomed to monitoring patients starting treatment with a S1P receptor modulator. Prof Centonze observed that, in practice, cardiac effects such as bradycardia are seen infrequently, even with fingolimod. He also noted that simplification of monitoring requirements, as is the case with the second-generation S1P receptor modulators, has been particularly advantageous during the COVID-19 era, when doctors have sought to minimise the use of drugs requiring patients to attend hospital for treatment. Indeed, prescriptions of fingolimod and other drugs requiring hospitalisation (monocloncal antibodies such as natalizumab, rituximab, alemtuzumab, or ocrelizumab) have decreased during the pandemic.
Prof Centonze concurred that second-generation S1P receptor modulators appear to offer an improved safety profile, speculating that ozanimod could prove to be a ‘gentle’ fingolimod with respect to safety. He noted that while extensive safety data have accumulated for fingolimod, additional long-term safety data are needed for the newer S1P receptor modulators, including data from ongoing extension trials. In the DAYBREAK16 extension study (Table 1), approximately 2,500 patients have received or continue to receive open-label treatment with ozanimod at the licensed dosage of 0.92 mg daily for up to 7 years. This study, set to be completed in 2022, will provide extensive long-term safety data for ozanimod. Interim analyses, in which patients had a mean total treatment duration of over 4 years on ozanimod, showed long-term safety and efficacy consistent with that seen in the parent trials.21
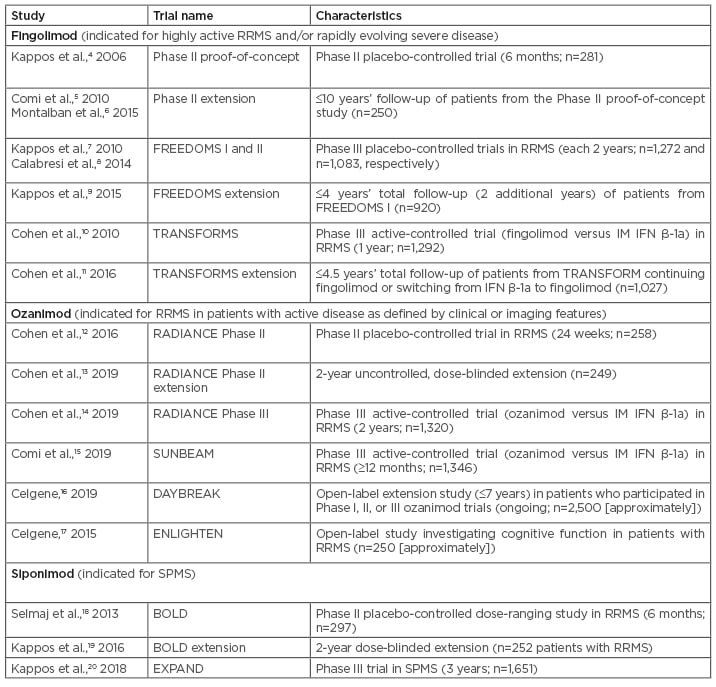
Table 1: Major sphingosine 1-phosphate receptor modulator clinical trials in multiple sclerosis.
IM: intramuscular; RRMS: relapsing-remitting multiple sclerosis; SPMS: secondary progressive multiple sclerosis.
EFFICACY OF S1P RECEPTOR MODULATORS: CLINICAL OUTCOMES, COGNITION, AND BRAIN VOLUME
There are no head-to-head trials that would permit evaluation of the comparative efficacy of different S1P receptor modulators. However, Prof Centonze drew some parallels between clinical trials of fingolimod and ozanimod. The pivotal ozanimod trials RADIANCE14 and SUNBEAM15 were active-controlled trials that used intramuscular IFN β-1a (Avonex® [Biogen, Cambridge, Massachusetts, USA]) as an active comparator (Table 1). The fingolimod TRANSFORMS10 trial also used intramuscular IFN β-1a as an active comparator, and enrolled patients with similar clinical characteristics to the RADIANCE and SUNBEAM trial populations. Based on these comparable trials, Prof Centonze speculated that ozanimod appears to be as effective as fingolimod.
Prof Ziemssen commented that real-world studies can be more telling than clinical trials in revealing the true efficacy and safety profile of a drug, reflecting on factors such as adherence and compliance outside the trial setting, and noted that he would like to see real-world data on the newer S1P receptor modulators. He mentioned the OZEAN study, an ongoing observational study following a cohort of patients receiving ozanimod treatment. No results from this study have been published to date, but findings are awaited with interest. Commenting on efficacy data from the RADIANCE and SUNBEAM trials, he highlighted the significant effect of ozanimod on relapse rates compared with IFN β-1a.14,15 He also mentioned that the trials included interesting endpoints that looked at various MRI measures and cognition.
Prof Ziemssen thought it was positive that ozanimod trials have placed more emphasis on cognition as an important component of MS. Although, he noted that cognitive assessment was primarily based on the Symbol Digit Modalities Test (SDMT), an interesting indicator of effects on cognition based on information processing speed, which does not provide a complete evaluation of cognitive function. He described the ozanimod data on cognition as a ‘nice appetiser’ but more comprehensive cognitive testing in future trials is required, as is research combining cognitive testing with neuroimaging. Cognitive decline is an under-recognised symptom of MS, in Prof Ziemssen’s opinion, and it would be beneficial to see more real-world monitoring of cognitive symptoms in clinical settings. Prof Centonze agreed that cognition, and mood, are important aspects of the disease, but pointed out the challenges of systematically assessing cognitive symptoms within MS treatment centres. Clinical trial data are therefore important in demonstrating the benefits on cognition and informing treatment choices.
Clinical trials are also a source of data on brain volume, which is not routinely measured in clinical practice, as Prof Centonze noted. It is thought that regional brain volume measurements may be more informative than cruder measurements of whole brain volume to help differentiate between DMT, as Prof Ziemssen highlighted, all high-efficacy DMT help to prevent whole-brain atrophy. The RADIANCE and SUNBEAM trials included measures of cortical and grey matter atrophy. Numerical decreases in both cortical grey matter and thalamic volume were seen with ozanimod, relative to losses in patients receiving IFN β-1a.14,15
Prof Centonze highlighted the potential central nervous system (CNS) effects of S1P receptor modulators, which cross the blood–brain barrier and may therefore be able to exert direct effects within the CNS, beyond any central effects that occur secondary to peripheral immunological changes. In the peripheral compartment, S1P receptor modulation leads to sequestration of T and B cells in the lymph nodes. This is a key aspect of the S1P receptor modulator mechanism of action, but it is not clear whether the degree of lymphocyte depletion correlates with clinical efficacy, or the extent to which other actions contribute to efficacy. Prof Ziemssen cited clinical research by his group that demonstrated reductions in neurofilament light chain, a marker of axonal damage, in the cerebrospinal fluid of patients treated with fingolimod. This research suggested that effects on neurofilament light chain in the CNS compartment could be a marker for fingolimod efficacy.22 Prof Centonze described preclinical studies with S1P receptor modulators, including research from his laboratory with both siponimod23 and ozanimod,24 that indicated that S1P receptor modulators appear to act in the CNS to modulate microglial cells. Further research is required to confirm specific CNS effects of ozanimod.
THE ROLE OF S1P RECEPTOR MODULATORS IN RELAPSING-REMITTING MULTIPLE SCLEROSIS: FUTURE DIRECTIONS
Prof Centonze outlined how he envisaged using the different S1P receptor modulators across the spectrum of patients at different stages of RRMS. For patients with early MS, he would use ozanimod, which he felt could be considered a first-line drug. Prof Centonze anticipated that the approval of ozanimod may prove very important, as it could result in a move towards earlier treatment using more potent drugs in patients with MS. Many existing first-line drugs have moderate efficacy; approval of ozanimod will allow early MS patients to receive high-efficacy treatment with an S1P receptor modulator. If first-line treatment had failed to prevent relapses or disease progression, Prof Centonze said he would continue to use fingolimod at this stage, because it currently has the strongest evidence base for use in the switch setting. Ozanimod could have similar efficacy to fingolimod in these patients, but has not yet accumulated as much evidence as fingolimod (although DAYBREAK includes patients switching from IFN β-1a in parent studies to ozanimod in the extension study21). Siponimod, another recently approved next-generation S1P receptor modulator, does have Phase II trial data in RRMS,19 but is approved only for secondary progressive MS.
“The approval of ozanimod will [prove to] be very important, as it will result in a move towards earlier treatment of MS patients with more potent drugs.” – Prof Centonze
Prof Centonze described the classic patient for whom he would consider ozanimod as young and treatment-naïve with low-level disability; effective early treatment in such patients is the best way to ensure a good clinical response. This patient profile matches that of a patient who could be considered a suitable candidate for dimethyl fumarate ([DMF] Tecfidera® [Biogen]), a twice-daily oral treatment likely to be the main competitor to S1P receptor modulators in early MS. Teriflunomide (Aubagio® [Sanofi Genzyme, Cambridge, Massachusetts, USA]) is another option for first-line oral treatment of MS, but DMF is more widely prescribed (in Prof Centonze’s experience). Prof Centonze indicated that he would expect an S1P receptor modulator to perform similarly to DMF in patients with early MS with regard to its efficacy, although tolerability profiles differ. He cited a propensity score-matched analysis in which DMF and fingolimod produced similar outcomes in suppression of disease activity in treatment-naïve patients.25 A treatment difference emerged in patients with higher levels of disease activity; fingolimod was superior to DMF in patients who were switching from self-injectable drugs,25 supporting its use in the switch setting, as discussed above.
In the wider MS treatment landscape, the potential approval of ofatumumab, which was approved by the U.S. Food and Drug Administration (FDA) in August 2020 for the treatment of relapsing forms of MS and was submitted for EMA approval in the same year, could lead to a broadening of the role of the anti-CD20 class. This would be analogous to the broadening of the S1P receptor modulator class with the approval of ozanimod, as Prof Centonze suggested ofatumumab could be a ‘gentler’ alternative to the first-in-class anti-CD20 agent ocrelizumab. In both classes, the new agents will provide neurologists with more options to use high-efficacy DMT in patients with early MS. Selection of one class over the other in the first instance would depend on individual patient profiles, both experts concurred. Prof Centonze noted that disease rebound has been observed when fingolimod was prescribed after discontinuation of ocrelizumab, and therefore caution should be exercised if switching to treatment with a S1P receptor modulator after initial anti-CD20 therapy.
Discussing the treatment of patients with early MS, Prof Ziemssen also advocated early use of high-efficacy treatment. He favoured a proactive approach to preventing progression of disability, stating that if a decision is taken to start with a lower efficacy treatment and escalate to higher efficacy options in reaction to further disease activity, it may then be too late to prevent accumulation of disability. In Germany, the only treatments licensed as first-line treatments were, until recently, the injectable treatments (IFN or glatiramer acetate), teriflunomide, and DMF. Fingolimod is not licensed as a first-line treatment option, and Prof Ziemssen suspected that the availability of an alternative S1P receptor modulator, i.e., ozanimod, that can be used as a first-line treatment, even after a patient’s first relapse, would prove advantageous, particularly for patients with negative prognostic markers such as significant MRI activity (e.g., >10 lesions on the patient’s first scan). MRI is an important tool to identify patients who would most benefit from first-line treatment with a high-efficacy DMT like ozanimod, by detecting potential subclinical disease activity, associated with poor long-term prognosis if allowed to progress. Prof Ziemssen also suggested that clinical activity, such as relapses with incomplete recovery, two relapses in close succession, or high baseline Expanded Disability Status Scale (EDSS) score, would also be indicators for high-efficacy treatment.
“In a disease like MS where we want to prevent disability [progression], we have to be proactive, and to have such a drug [as ozanimod] available for first-line treatment of recently-diagnosed patients who have active MS will make an important difference.” – Prof Ziemssen
CONCLUSION
Both Prof Ziemssen and Prof Centonze spoke positively about S1P receptor modulators as a class, and about recent additions to the class that provide the potential to expand the use of S1P receptor modulators to a broader range of MS patients. Their comments suggest that there is a clear role for ozanimod alongside fingolimod for the treatment of active RRMS, and the approval of ozanimod as a treatment option with a favourable safety profile, which can be used in first- and later-lines, will prove very important, enabling more patients to benefit from early treatment with a high-efficacy oral DMT.

![EMJ Neurology 8 [Supplement 2] 2020 Feature Image](https://www.emjreviews.com/wp-content/uploads/2020/12/EMJ-Neurology-8-Supplement-2-2020-Feature-Image-940x563.jpg)






