Abstract
Background: The use of conventional flexible forceps during flex-rigid pleuroscopy can be challenging when sampling hard and thickened pleura. Pleuroscopic cryobiopsy is an emerging field, with various early studies demonstrating good yield and minimal complications.
Objectives: To review the authors’ early experience of pleuroscopic cryobiopsy in their centre and highlight its utility in the diagnosis of mesothelioma.
Method: Six cases of undiagnosed pleural effusion that underwent pleuroscopic cryobiopsy via flexi-rigid pleuroscopy between July 2017 and June 2019 were retrospectively analysed.
Results: The cohort had a median age of 59 years, consisting of two females and four males with a median age of 57 years. Mean (aggregate) tissue sample diameter was 9.2±1.9 mm. Cryobiopsy established a definitive diagnosis in all six cases: one case of malignant mesothelioma, one pleural tuberculosis, two small cell carcinomas, and two adenocarcinomas. Immunohistochemical staining was performed in all five of the malignant samples (100%). There were no major complications reported.
Conclusions: Based on the case series, pleuroscopic cryobiopsy is a feasible adjunct to conventional forceps biopsy, a safe procedure in the authors’ setting, gives high diagnostic yield, and enables differentiation between the different causes of exudative pleural effusion. A large, prospective study is required to validate this retrospective series.
INTRODUCTION
Following diagnostic thoracocentesis and closed pleural biopsy, 25% of pleural effusions remain undiagnosed,1 requiring either pleuroscopy or video-assisted thoracic surgery (VATS). While VATS is considered the gold standard, pleuroscopy is a less invasive, simpler, and cost-effective alternative with a favourable diagnostic yield.2 Furthermore, VATS is not always available, and is sometimes contraindicated, especially in patients with high surgical risk or advanced metastatic malignancy.2
Rigid pleuroscopy demonstrates higher yields of 97.8% versus 73.3% for flexi-rigid pleuroscopy due to larger sample sizes, superiority in sampling fibrous pleura in suspected mesothelioma, and its effectiveness in adhesiolysis.3 On the other hand, advantages for flexi-rigid pleuroscopy include less pain and easier maneuverability.4,5 Efforts to increase yield of flexi-rigid pleuroscopy has led to the use of cryobiopsy, a technique previously validated for endobronchial and transbronchial biopsies.6 Cryobiopsy through flexi-rigid pleuroscopy is an emerging technique which carries the promise of both larger sample size with preserved tissue architecture, while maintaining ease of manoeuvrability with a flexible pleuroscope.
In the Southeast Asia region, the incidence of mesothelioma is lower compared with European or Oceanian countries. Many benign pleural diseases mimic similar histopathological changes in mesothelioma;7 therefore, proper acquisition of tissue is important in Malaysia to ensure a diagnosis of mesothelioma is not missed.
MATERIALS AND METHODS
Six patients with malignancy-suspicious pleural effusion who underwent pleuroscopic cryobiopsy at the authors’ unit between July 2017 and June 2019 were analysed. In that period, a total of 244 patients underwent pleuroscopic biopsy: 14 via rigid pleuroscope, 224 via flexi-rigid pleuroscope using conventional forceps, and six via flexi-rigid pleuroscope using cryobiopsy. Written informed consent was obtained from all patients. Pleural fluid analysis was performed via ultrasound-guided diagnostic thoracocentesis prior to the procedure. Baseline blood investigations including coagulation profile were taken for each patient and ensured normal prior commencing the procedure.
Transthoracic ultrasound was performed on each patient to identify the safe entry site with the patient in lateral position with the abnormal side upwards. The procedure was performed under conscious sedation with intravenous midazolam and pethidine. Under aseptic technique, the entry site was infiltrated with local anaesthesia (2% lidocaine). Flexible electrocautery equipment were prepared for any potential bleeding complications.
A 1.5 cm incision was then made on the skin and blunt dissection was performed until the pleura was breached. A 11 mm metallic trocar was then inserted into the pleural cavity for pneumothorax induction. Pleuroscopy was performed using a flexi-rigid scope (LTF-160, Olympus, Tokyo, Japan) passed through the trocar. All pleural fluid was aspirated until clear to allow clear examination of the pleural cavity. Pleural fluid was sent for further analysis if initial diagnostic thoracocentesis data was inadequate.
After cautious examination of the pleural cavity, cryobiopsies were obtained at visibly abnormal areas using a 950 mm cryoprobe of either 1.9 or 2.4 mm diameter passed through the working channel of the pleuroscope in conjunction with the ERBECRYO®2 system (Erbe Medizintechnik, Tübingen, Germany). Carbon dioxide was used as cryogen. The tip of the cryoprobe was applied to the target biopsy sites and activated for a minimum time of 3 sec under direct vision, and pleural specimens were removed by extracting the cryoprobe and flexi-rigid scope together through the trocar. The specimen was thawed in normal saline, placed into formalin solution, and immediately sent to the laboratory for histopathological analysis. Meanwhile, the pleuroscope was reinserted into the pleural cavity to assess for any bleeding or complication. The biopsy procedure was repeated for a minimum of three times, given that no complications occurred. The decision to employ cryobiopsy was at the discretion of the endoscopist when specimen from forceps biopsy was deemed inadequate during intraprocedure.
CASE SERIES
Case 1
Case 1 was a 64-year-old indigenous Malaysian female who did not smoke and had no underlying medical illness. She presented with 1 week of progressively worsening shortness of breath on a background of 1 month of persistent non-productive cough and loss of appetite. When asked, she did not report any fever or night sweats. Clinically, she was not tachypnoeic but had finger clubbing and reduced air entry over the left lower zone of the chest. Her vitals were unremarkable with a temperature of 36.6 oC and oxygen saturation of 97% under room air. Chest radiograph showed blunting of the left costophrenic angle and ultrasound of the thorax confirmed a left non-loculated pleural effusion. Her total white cell count was 6.7×109 cells/L. Pleural fluid parameters were exudative (pH = 7.355; pleural fluid:serum protein ratio = 50/72 g/L; pleural fluid:serum lactate dehydrogenase [LDH] ratio = 1554/426 U/L; fluid glucose = 4.8 mmol/L); pleural fluid cytology showed no malignant cells. Pleuroscopy revealed multiple pleural and diaphragmatic nodules; cryobiopsy was performed with no major complication. Talc pleurodesis was applied prior to removal of the chest drain. Histopathological and immunohistochemical examination confirmed epidermal growth factor receptor-positive lung adenocarcinoma, and she was referred to the oncology team for commencement of tyrosine-kinase inhibitor therapy.
Case 2
Case 2 was a 59-year-old Malay male with a 50-pack per year smoking history, underlying hypertension, and dyslipidaemia, who presented with 1 week of right lateral chest pain and shortness of breath on a background of chronic dry cough of 2 months, accompanied by loss of weight and appetite. He did not have a history of fever. On clinical examination, he was tachypnoeic with a respiratory rate of 24 breaths per minute and oxygen saturation of 92% under room air. He had no finger clubbing; chest examination demonstrated reduced air entry over the right lower and middle zones. His temperature was 36.5 oC and his total white cell count was 7.7×109 cells/L. Chest radiograph showed a right homogenous opacity with meniscus sign obliterating the right heart border. Ultrasound thorax confirmed a non-loculated right pleural effusion. Pleural fluid parameters were exudative (pH = 7.531; pleural fluid:serum protein ratio = 42/72 g/L; pleural fluid:serum LDH ratio = 722/894 U/L; pleural fluid glucose = 5.3 mmol/L); pleural fluid cytology showed no malignant cells. Pleuroscopy showed multiple visceral and parietal pleural nodules; cryobiopsy was performed with no major complication. Talc pleurodesis was applied prior to removal of the chest drain. Histopathological and immunohistochemical examination confirmed small cell carcinoma of the lung, and he was referred to the oncology team for further management.
Case 3
Case 3 was a 60-year-old indigenous Malaysian female who did not smoke and had a history of right breast carcinoma in 1999, for which she had a right mastectomy and subsequently had been in stable remission. In December 2017, she presented with 2 weeks of shortness of breath and right lateral chest discomfort for 3 days, but, when asked, did not report any fever, cough, night sweats, or overt loss of weight. On clinical examination, she had a respiratory rate of 23 breaths per minute, temperature of 36.2 oC, and oxygen saturation of 90% under room air, with chest examination demonstrating reduced air entry over the entire right thorax. Her total white cell count was 8.3×109 cells/L. Chest radiograph revealed a ‘white-out’ appearance of the right lung. Ultrasound thorax confirmed a very large non-loculated right pleural effusion. Pleural fluid parameters were protein-discordant exudative (pH = 7.497; pleural fluid:serum protein ratio = 34/74 g/L; pleural fluid:serum LDH ratio = 472/400 U/L; pleural fluid glucose = 3.6 mmol/L); pleural fluid cytology was inconclusive, showing malignant cells. Pleuroscopy showed multiple diaphragmatic nodules; cryobiopsy was performed with no major complication. Talc pleurodesis was administered prior to chest tube removal. Histopathological and immunohistochemical examination confirmed metastatic adenocarcinoma secondary to her primary breast malignancy. She underwent palliative treatment under the oncology team. As of 18 months after the cryobiopsy, surveillance CT did not show further progression of disease.
Case 4
Case 4 was a 67-year-old Malay male who did not smoke but did have underlying Type 2 diabetes mellitus and was on insulin treatment. He presented in January 2019 with complaints of fever, shortness of breath, and loss of appetite for 2 weeks, associated with right lateral chest discomfort. He did not report any chronic cough or haemoptysis. On clinical examination, he had a respiratory rate of 22 breaths per minute, temperature of 37.1 oC, and oxygen saturation of 95% under room air, with chest examination showing reduced air entry over the right lower zone. His total white cell was 6.8×109 cells/L. Chest radiograph showed a right homogenous opacity with meniscus sign partially obliterating the right heart border. Ultrasound thorax revealed a non-loculated pleural effusion. Pleural fluid parameters were exudative (pH = 7.466; pleural fluid:serum protein ratio = 59/78 g/L; pleural fluid:serum LDH ratio = 330/612 U/L; pleural fluid glucose = 10.38 mmol/L); pleural fluid cytology showed predominantly lymphocytic effusion. Sputum examination for acid-fast bacilli was negative. Pleural fluid and sputum culture for tuberculosis were negative. Pleuroscopy revealed multiple parietal pleural nodules, several of which were tangentially oriented; cryobiopsy was performed with no major complication. No talc pleurodesis was given in view of suspected tuberculous infection. Histopathological and immunohistochemical examination confirmed caseating granulomas; the patient was started on tuberculosis treatment, after which clinical improvement in terms of weight gain, symptom resolution, and radiological improvement as evidenced by eventual resolution of the effusion on chest radiographs were demonstrated.
Case 5
Case 5 was a 58-year-old Malay male who smoked but had no underlying medical illness. He presented in June 2019 with a 3-month history of chronic cough and loss of weight and appetite. He did not report fever and haemoptysis. Clinically, he had a respiratory rate of 24 breaths per minute, temperature of 37.0 oC, and oxygen saturation of 93% under room air. He was clinically clubbed, and chest examination revealed reduced air entry over the left lower and mid zone. Chest radiograph showed a left homogenous opacity with meniscus sign and obliteration of the left heart border. Ultrasound of the thorax showed a non-loculated left pleural effusion. His total white cell count was 9.9×109 cells/L. Pleural fluid parameters were exudative (pH = 7.394; pleural fluid:serum protein ratio = 45/77 g/L; pleural fluid LDH = 207 U/L; pleural fluid glucose = 6.8 mmol/L); pleural fluid cytology showed no malignant cells. Pleuroscopy revealed multiple pleural and diaphragmatic nodules; cryobiopsy was performed with no major complication. Talc pleurodesis was applied prior to removal of the chest drain. Histopathological and immunohistochemical examination confirmed small cell carcinoma of the lung. He was referred to the oncology team for further care.
Case 6
Case 6 was a 71-year-old indigenous Malaysian male who smoked and had a history of asbestos exposure. He presented in June 2019 with a 2-month history of progressive shortness of breath and dry cough, associated with loss of weight. He did not report fever or haemoptysis. Clinically, he had a respiratory rate of 23 breaths per minute, temperature of 36.8 oC, and oxygen saturation of 91% under room air. Chest examination revealed reduced air entry over the right lower and midzone. Chest radiograph revealed a right homogenous opacity with meniscus sign and obliteration of the right heart border. Ultrasound of the thorax showed a non-loculated right pleural effusion. His total white cell count was 7.9×109 cells/L. Pleural fluid parameters were exudative (pH = 7.258; pleural fluid:serum protein ratio = 42/73 g/L; pleural fluid:serum LDH ratio = 954/321 U/L; pleural fluid glucose = 5.1 mmol/L); pleural fluid cytology was inconclusive, showing atypical cells. Pleuroscopy showed scattered small hard nodules; cryobiopsy was performed with no major complication. Talc pleurodesis was applied prior to removal of the chest drain. Histopathological and immunohistochemical examination confirmed malignant mesothelioma, and the patient was referred to the oncology team for further management.
RESULTS
Baseline Demographic Data
A total of six patients of either indigenous Malaysian or Malay descent underwent pleuroscopic cryobiopsy (Table 1). Two were female and four were male, with a median age of 59 years (interquartile range: 56–67).
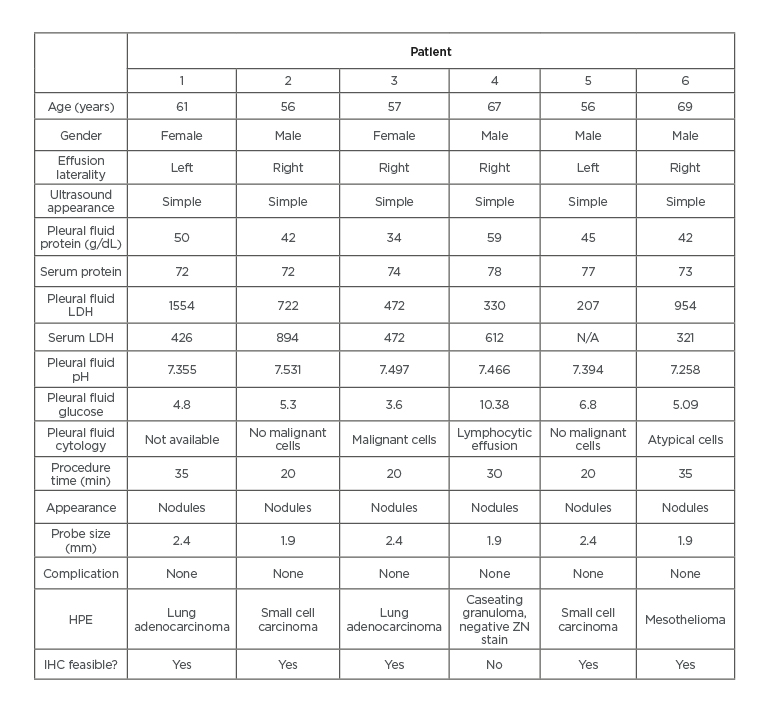
Table 1: Characteristics of pleuroscopic cryobiopsy patients.
HPE: histopathological examination; IHC: immunohistochemistry; LDH: lactate dehydrogenase; ZN: Ziehl–Neelsen.
Characteristic of Pleural Effusion
Two (33.3%) of the effusions were left-sided, while four (66.7%) were right-sided. All effusions had a simple appearance on ultrasound.
Three (50.0%) patients had effusions occupying one-half of the hemithorax, two patients (33.3%) had effusions occupying less than one-half of the hemithorax, and one (16.7%) had a massive white-out effusion. Pleural fluid pH was elevated in Cases 2, 3, and 4. Five of the effusions were exudative, one was protein-discordant (Case 3). Median pleural fluid protein was 43.5 g/dL while median LDH was 597 IU/L. Pleural fluid cultures were negative in all samples. Cytology was inconclusive in all six specimens, with only one sample showing malignant cells (Case 3) and another showing atypical cells (Case 6).
Pleuroscopic Characteristic
All six cases (100%) had a nodular pleuroscopic appearance. A 1.9 mm probe was used in three cases (50%), while a 2.4mm probe was used in the other three cases (50%).
Median activation time and the number of exact activations were not recorded in the patients. The mean aggregate sample size was 9.2±1.9 mm (Table 2).4,8-12 A definite histopathological diagnosis was obtained in all six cases. Five (83.3%) patients had confirmed malignancy (one adenocarcinoma lung, one adenocarcinoma breast, two small cell carcinomas, and one mesothelioma) and one (16.7%) patient was diagnosed with pleural tuberculosis. The five (83.3%) malignant samples were adequate for immunohistochemical staining. None of the six patients encountered major complications. The median procedural time was 25 minutes (range: 20–35 minutes).
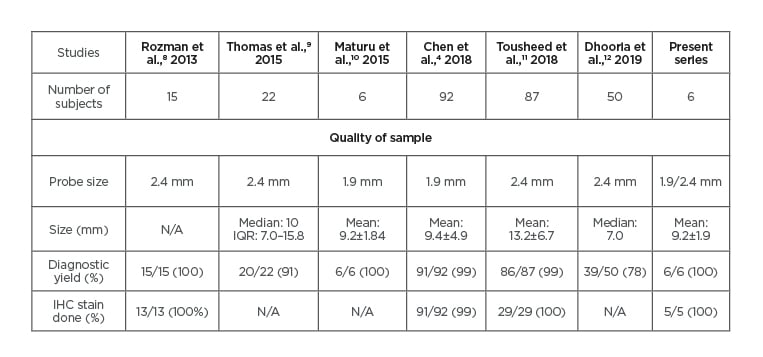
Table 2: Comparison to previous studies of pleuroscopic cryobiopsy.
IHC: Immunohistochemistry; IQR: interquartile range; N/A: not available.
DISCUSSION
Cryobiopsy is a technique that employs the use of a blunt probe cooled by nitrous oxide or carbon dioxide, which draws moisture out of surrounding tissue and freezes with it by creating a bond (Joule–Thomson effect).13 In pleuroscopic biopsy, cryobiopsy was historically performed with a rigid probe via a second trocar, under direct rigid pleuroscopy examination.13 Recent technological advancements have led to the flexible cryoprobe which can be inserted through the working channel of flexible endoscopes. The use of cryobiopsy in bronchoscopy has been validated in multiple studies for endobronchial biopsies and transbronchial biopsies with minimal additional bleeding risk.6 Meanwhile, studies on cryobiopsy via flexi-rigid pleuroscopy have only recently burgeoned in the past decade, with studies citing comparable sample sizes and yield, preserved biopsy sample architecture allowing for immunohistochemical studies, and similar safety profile compared to conventional forceps biopsy via flexi-rigid pleuroscope.4,5,8-12,14,15 To the authors’ best knowledge, this is the first case series of pleuroscopic cryobiopsy in the Southeast Asia region.
Pleural fluid biochemical characteristics are poor differentiators of tuberculous and malignant pleural effusions,16 as demonstrated in this case series. The higher pH values in Cases 2, 3, and 4 were likely due to exposure to air during collection via the suction port of the scope, causing artificial elevation of pH.17 Pleural fluid cytology carries overall sensitivity of 67.2% in malignant pleural effusion of all types; of these, the sensitivity was 87.9% for adenocarcinomas but dropped to 45.5% in malignant mesothelioma.18 In this series, none of the pleural fluid cytology results were conclusive; only Cases 3 and 6 had abnormal fluid cytology showing malignant and atypical cells, respectively, which were later confirmed via cryobiopsy as adenocarcinoma and mesothelioma (Table 1). While the diagnostic accuracy of cytology in malignant mesothelioma has been shown to increase with the availability of an experienced cytopathologist in a region of high prevalence for mesothelioma,18 this may not be applicable in the Southeast Asia region. This highlights the unreliability of pleural fluid cytology and the need for a definitive biopsy to clinch the diagnosis. While sensitivity and specificity of flexi-rigid pleuroscopy using conventional forceps in undiagnosed exudative pleural effusion is high,5 increased false-negative results are seen in cases of thickened pleura.19,20 Some factors that contribute to this include inadequate depth of biopsy sample20 and the lack of mechanical power of the conventional forceps and a small cup size.4,5
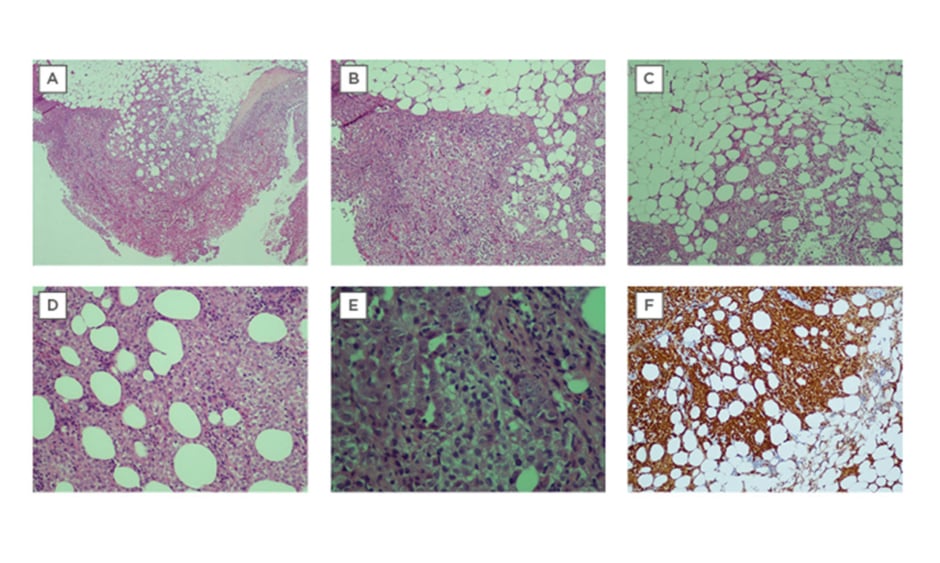
Cryobiopsy poses a potential solution to this problem, having been shown to be non-inferior to conventional forceps in the diagnosis of malignant mesothelioma.21 The crucial advantage lies in that it can acquire significantly greater depths of tissue than conventional forceps;4,9,10,12 one study reported extrapleural fat in 65.2% of cryobiopsy samples as opposed to 40.8% in conventional forceps biopsy.12 This was demonstrated in Case 6 (Table 1), whereby the patient’s diagnosis of malignant mesothelioma was confidently obtained due to the demonstration of extrapleural fat, which is essential to visualise invasion of abnormal mesothelial cells in mesothelioma (Figure 1). In Southeast Asia, reported rates of non-specific pleuritis range from 12.0%22 to 21.6%.23
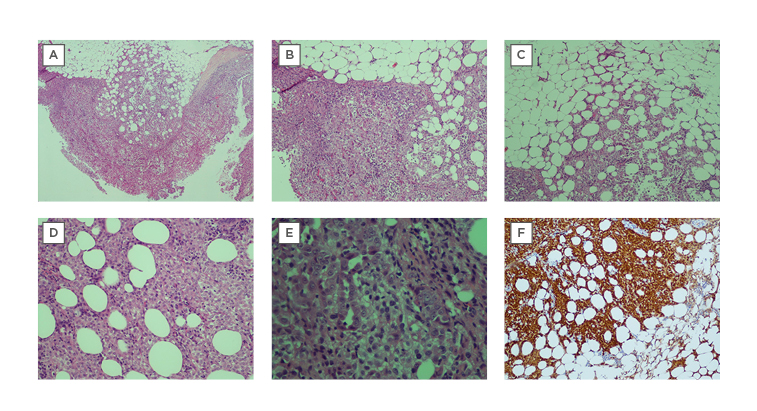
Figure 1: Cryobiopsy sample for Case 6 was conclusive for malignant mesothelioma.
A) Extrapleural adipose tissue is well demonstrated and there are no artefacts. There is marked increase of atypical mesothelial cells proliferation superficially, with infiltration into the underlying adipose tissue, which is indicative of malignancy (x40) H&E stain. B) The tumour cells are arranged in sheets (x200) H&E stain. C) Tumour cells are seen infiltrating into extrapleural adipose tissue (x200) H&E stain. D) Tumour cells infiltrating in between adipose cells (x400) H&E stain. E) The tumour cells show round vesicular nuclei with prominent nucleoli. Tubular formation is also noted. F) Immunohistochemical staining was positive for calretinin (d: ×200)
H&E: haemotoxylin and eosin.
Although long-term follow-up on these cases did not frequently reveal a diagnosis of mesothelioma,20 it is prudent to consider the possibility that the low prevalence of mesothelioma in Southeast Asia may be partly contributed by shallow biopsies leading to inconclusive or alternative diagnoses. Further research could look into the value of cryobiopsy in non-specific pleuritis.
Moreover, certain varieties of mesothelioma such as desmoplastic mesothelioma make conventional biopsy extremely difficult because they are often smooth and non-nodular macroscopically. In such cases, one author has proposed a combination of incisions via electrocautery technique followed by cryobiopsy.21 Another author has suggested creating a break in the pleura with forceps prior to cryobiopsy,9 but this has to be weighed against the risk of bleeding. The present authors have yet to implement these combined methods in their pleuroscopic setting; further studies are needed to validate them.
The second advantage of the cryoprobe is its ability to biopsy samples from lesions positioned tangentially to the instrument.4,10 As opposed to conventional forceps, which only allow forward sampling, cryoprobe can obtain tissue in a 360o manner laterally.24 Similarly, in the experience with Case 4, nodules that were tangentially oriented despite having the pleuroscope at maximal angulation were much easier to sample with cryoprobe than conventional forceps. Third, in terms of technical difficulty, there is no significant difference in operator-rated difficulty when comparing cryobiopsy to conventional forceps technique;12 this is promising and may encourage more pulmonologists to adopt it in the future, although studies on its learning curve are lacking. The authors’ limited experience with pleuroscopic cryobiopsy was not a major deterrent to obtaining accurate diagnosis, as evidenced by good sample sizes comparable to other larger studies, and successful histopathological diagnosis in all six of their patients (Table 2).
The most dreaded complication of cryobiopsy is always major bleeding, which was not encountered in these six cases (Table 1). Early studies have not described any significant risks of major bleeding, but various authors have advocated gentle withdrawing of the probe as essential to prevent severe haemorrhage.4,8-11 Furthermore, one author opined that in cases when the probe was already attached to the tissue but could not be gently removed, the probe should be allowed to unfreeze and resume with a shorter period of freezing.8 Freezing time of the cryoprobe should be variable, to be adjusted throughout the procedure, as to not take tissue samples deeper than the muscle layer to reduce the risk of bleeding or nerve injury.21
One of the limitations of the authors’ study is that biopsy was only performed on visibly abnormal areas for at least three passes. Expert recommendations advocate at least five biopsies carried out over abnormal and normal pleura to ensure adequate tissue quality and quantity.25,26 However, these recommendations were made in regard to conventional forceps rather than cryobiopsy. In Case 6, three cryobiopsies were performed, comparable to prior studies which performed a range of one to four cryobiopsies per patient.4,8-11,14,16 The optimal number of cryobiopsies needed to balance yield and the risk of bleeding remains uncertain, this is a potential area for future research.
Another limitation of this study was that pleural biopsy for tuberculosis culture was not routinely sent. The gold standard of diagnosing pleural tuberculosis is the isolation of Mycobacterium tuberculosis in either pleural fluid or pleural tissue by culture, or demonstration of caseating granulomas in histology.27 Tuberculosis culture is important for drug sensitivity testing, but has a sensitivity of only 68.7% as opposed to 100.0% in pleuroscopy.28 Furthermore, there is often a delay in waiting for culture results. In tuberculous endemic regions at the hand of an experienced investigator, tuberculous pleurisy usually presents with characteristic inflammatory patterns on pleuroscopy, such as caseous, sago-like nodules, or fibrin deposits and loculations.29 As demonstrated in Case 4, representative appearance on pleuroscopy combined with histological finding of granuloma was sufficient to clinch the diagnosis of pleural tuberculosis. A demonstrated therapeutic response to antituberculous medication further reinforced the diagnosis of pleural tuberculous in this cohort of patients.
CONCLUSION
In conclusion, pleuroscopic cryobiopsy was feasible with no major complications in the six subjects; samples were comparable in size to those in previous studies and had preserved tissue architecture, allowing for histopathological confirmation of diagnosis and immunohistochemistry studies. Pleuroscopic cryobiopsy may prove useful both in the diagnosis of malignant mesothelioma in regions of low prevalence or in centres where cytological analysis is unreliable or unavailable.
STATEMENT OF ETHICS
Subjects have given their written informed consent for publication of this case report and accompanying images. This article does not contain any studies with human participants or animals performed by any of the authors. The authors have no ethical conflicts to disclose.