Meeting Summary
The main objectives of the symposium were to review recent evidence on what difference targeting Psoriasis Area Severity Index (PASI) 90 or 100 and Dermatology Life Quality Index (DLQI) 0 or 1 treatment outcomes, or targeting the IL-17 cytokine or receptor, make to patients with psoriasis and whether our current approaches are ambitious enough. Prof Griffiths introduced the symposium and discussed the importance of recognising that psoriasis is stigmatising for patients and that clear skin plays a major role in reducing the burden of disease. Prof Griffiths then provided an overview of approaches to assessing psoriasis disease severity, such as PASI, and described recent clinical efficacy data indicating that a treatment outcome of PASI 90 and even PASI 100 response is a realistic aim. Dr Chiricozzi explained the evidence for the role of the IL-17 cytokine family in psoriasis pathogenesis and inflammation and how the only therapeutic strategy to simultaneously block all the inflammatory signals stimulated by IL-17 cytokines is blockade of the IL-17 receptor subunit A (IL-17RA). Finally, Prof Augustin discussed the importance of patient-reported outcomes (PRO) in obtaining the patients’ perspective on the value of treatment. He described the use of DLQI in practice and summarised findings from real-world studies that demonstrated that DLQI 0 or 1 highly reflects patient benefit from treatment.
Does it Make a Difference and Why Should You Care?
Professor Christopher Griffiths
Patients with psoriasis still face stigmatisation and consequently use ‘avoidance coping’ to try and reduce the stigma they experience. The psychosocial impact of psoriasis is considerable, with patient-reported physical outcomes comparable or slightly worse for psoriasis than those for diabetes, arthritis, heart disease, depression, and cancer.1 Therefore, discussing raising the bar for treatment outcomes, such as PASI 90 or 100 and DLQI 0 or 1 responses, is important so that we may aim to achieve the greatest benefit for patients.
Although PASI 75 is the current gold standard treatment outcome with new treatments, such as IL-17 inhibitors, complete skin clearance (PASI 100) should become a realistic goal for many patients. In an analysis of a real-world observational study (PSO-BIO-REAL) of patients with moderate-to-severe plaque psoriasis who were initiating or switching biologics, 23% and 26% of patients achieved PASI 100 at 6 months and 12 months, respectively.2 A slightly higher proportion of patients who were biologic-naïve compared to biologic-experienced (25% versus 20%, respectively, at 6 months) achieved PASI 100.2 While biologics, including IL-17 inhibitors, have demonstrated high levels of skin clearance in clinical trials,3 it remains to be established whether similar levels can be achieved in clinical practice and more effective treatments are needed.
In summary, it is important to recognise that psoriasis is stigmatising for patients and that clear skin plays a major role in reducing the burden of the disease. Therefore, there is a need to discuss optimal, ambitious, and holistic treatment of our patients.
What is the Difference between PASI 100 and PASI 90, and is PASI 100 a Realistic Treatment Goal in Daily Clinical Practice?
Professor Christopher Griffiths
There are several methods for assessing psoriasis severity. PASI assessment is now a standard measure and changes in this score are commonly used as treatment outcome measures. However, PASI is not a very accurate assessment of severity because it only considers erythema, desquamation, and induration, and the surface area involved according to anatomical sites, giving a total score ranging from 0–72. Given that a PASI score of >12 represents severe psoriasis, there is huge redundancy in the scale, with scores of >50 very rare. Additionally, dermatologists may not know what a PASI of 10, 20, or 30 looks like. Consequently, using a more holistic approach to assess psoriasis severity is needed. One such assessment is the Simplified Psoriasis Index,4 a summary measure consisting of three component aspects of psoriasis: current severity, current psychosocial impact, and a historical course and intervention score. The sum of these component scores shows whether a patient will be relatively straightforward or difficult to treat, as it not only includes the body surface area affected but is weighted towards more sensitive areas, such as the face or hands, and includes psychosocial disability and previous response to treatment.4
In terms of assessing response to treatment, two randomised clinical studies (AMAGINE-2 and 3) in patients with moderate-to-severe psoriasis evaluated PASI 100 as an endpoint for the comparison of brodalumab, an anti-IL-17 receptor antibody, and ustekinumab, an anti-IL-12/IL-23 antibody.5 In a post-hoc analysis of AMAGINE-2 and 3, the cumulative incidence of patients receiving brodalumab 210 mg every 2 weeks (Q2W) achieving PASI 100 in four body regions by Week 52 were 91% (head and neck), 90% (trunk), 86% (upper limbs), and 83% (lower limbs).6 This reflects what is seen in clinical practice, with the fastest response observed for the head and neck and the slowest for the lower limbs. In a further post-hoc analysis evaluating PASI <75, 75, 90, and 100 responses over time, the PASI 100 response rate for patients treated with brodalumab 210 mg Q2W increased over time to ~55%.7 Thus, the efficacy of new biologics indicates that we should realistically be aiming for an outcome of at least PASI 90 and even PASI 100. The change in absolute PASI scores may also be used to evaluate outcomes. In AMAGINE-2 and 3, the proportion of patients treated with brodalumab 210 mg Q2W who achieved a PASI score of 0 or >0 and ≤1 over time reached ~65%,7 providing evidence to further evaluate absolute PASI (Figure 1).
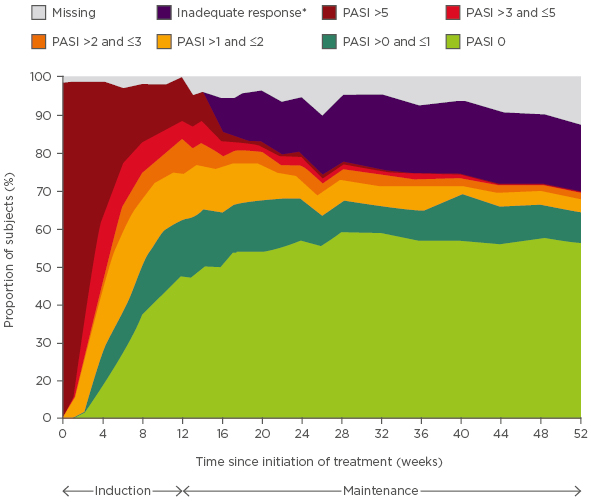
Figure 1: Proportion of patients over time with absolute Psoriasis Area Severity Index scores for brodalumab 210 mg every 2 weeks.
*Defined as static Physician’s Global Assessment (range 0–5) ≥3 or persistent values of 2 over at least a 4-week period at or after Week 16.
PASI: Psoriasis Area Severity Index.
Adapted from Zachariae et al.7
It is also important to consider what complete skin clearance means and to understand the mechanism and drivers at the molecular and immunological level of the characteristic relapses of psoriasis in the same sites. One concept is that of the ‘molecular scar’, whereby microscopic residual abnormalities with a predominance of psoriasis or disease-related genes remain, even in clinically resolved psoriasis lesions.8 At an immunological level, there are residual populations of tissue-resident memory T cells in clinically resolved lesions.9 These T cells respond to autoantigens that stimulate a psoriasis flare, then predominantly produce IL-17, which results in the recurrence of the lesion in the same site. This raises the possibility that, in addition to resolving the lesion, we should also aim to clear these residual T cells to reduce the risk of relapse.
Lastly, there is ongoing debate about whether dermatologists should see patients much earlier in the psoriasis disease cycle. Rapid referrals from primary care to specialists help ensure patients are on the correct treatment pathway and aim to prevent the sequelae of psoriasis by educating patients on the risk factors for comorbidities, such as cardiovascular disease, and screening for psoriatic arthritis.10 Additionally, the concept of treating some patients very early with new biologics, such as anti-IL-17 or anti-IL-23 antibodies, to see if they could prevent the continuance of residual T cells and thereby switch-off the disease and prevent relapses, should be investigated.
Where is the Difference in the IL Pathways? Does it Make a Difference Whether the Treatment Targets the Cytokine or the Receptor?
Doctor Andrea Chiricozzi
The IL-17 cytokine family plays an important role in psoriasis pathogenesis and inflammation and consists of six members from IL-17A to IL-17F.11,12 IL-17A and IL-17F can form both homodimers and IL-17A/F heterodimers. IL-17 cytokines signal through heterodimeric receptor complexes in the IL-17R family (Figure 2).11–13 IL-17A, IL-17C, IL-17E, and IL-17F all signal through the IL-17RA subunit;14 therefore, IL-17 RA represents a therapeutic target in psoriasis. The general biological activity and pathogenic role of IL-17B and IL-17D in psoriasis are not well understood and, as such, were not further discussed in this symposium.
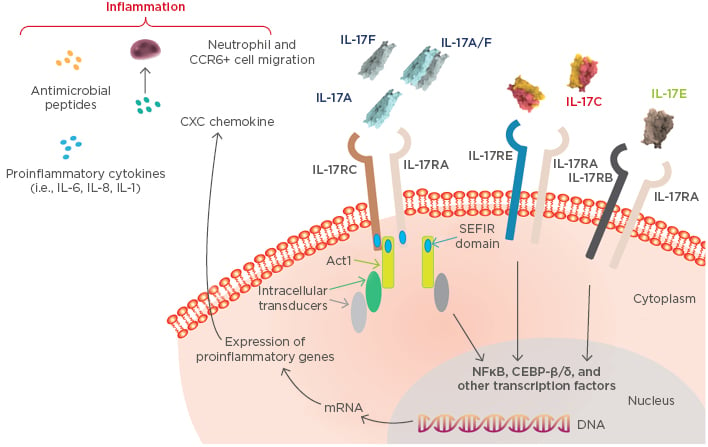
Figure 2: IL-17 cytokine family-mediated inflammatory pathways in psoriasis.
Adapted from Beringer et al.13
IL-17A
IL-17A is a central cytokine in psoriasis and, with IL-23, constitutes the main axis driving the development of the psoriasis phenotype.15 In this axis, IL-23 stimulates a wide array of immune cells to produce and express IL-17A, including Th17, Tc17, Tγ/δ+, natural killer, innate lymphoid, neutrophils, and mast cells.12,14 These cells infiltrate lesional psoriatic skin and produce IL-17A. Neutrophils are not likely to express IL-17A mRNA, but instead internalise IL-17A produced by other cells and, once activated, are able to release it.16 The infiltration results in increased expression of IL-17A that can be detected in lesional and non-lesional psoriatic skin compared to normal skin, as well as increases in IL-17A serum levels versus healthy controls and increases in IL-17 concentration in the tear liquid of patients with psoriasis.17-19
IL-17A is a proinflammatory cytokine that directly affects tissue cells, particularly keratinocytes.16 Keratinocytes are considered the key responding cells to the skin cytokine microenvironment and are important for inflammation induced in the skin. In keratinocytes, IL-17A stimulates the expression of proinflammatory mediators, such as antimicrobial peptides (e.g., lipocalin, S100A proteins, and beta defensins), and, in synergy with TNF-α, it stimulates the expression of proinflammatory cytokines (e.g., IL-1β, IL-6, IL-17C) and chemokines (e.g., IL-8 and CCL20). The stimulation by IL-17A results in feed-forward loops that sustain skin inflammation.20,21
In vitro experiments in a three-dimensional skin model showed that IL-17A can regulate the expression of >630 genes.22 Furthermore, IL-17A induced a gene expression profile that strongly correlated with the altered gene expression profile in lesional psoriatic skin,22 meaning that IL-17A is a good therapeutic target. For example, secukinumab23 and ixekizumab24 neutralise IL-17A in both the homodimer and heterodimer, resulting in selective inhibition that suppresses the inflammatory gene expression regulated solely by IL-17A.11,25,26 However, other IL-17 cytokines can contribute to inflammation in psoriasis.
IL-17F
IL-17F shares 55% sequence homology with IL-17A and is upregulated in lesional psoriatic skin compared to non-lesional and normal skin.27,28 Moreover, IL-17F is produced by Th17 cells that also produce IL-17A and its expression is regulated by IL-23.29-31 IL-17F homodimers and IL-17A/F heterodimers (and IL-17A homodimers) signal through the receptor consisting of IL-17RA and IL-17RC subunits (Figure 2).12,13 Biologically, IL-17F almost overlaps with IL-17A, stimulating genes similar to those stimulated by IL-17A. In a recent study, similar gene expression signatures were induced in human skin explants treated with IL-17A, IL-17F, and IL-17A/F heterodimers.32 Thus, IL-17F can induce gene expression of the same antimicrobial peptides, cytokines, and chemokines that have been previously described for IL-17A.32 While there is an overlap in IL-17A and IL-17F signalling, IL-17A is 10–30-fold more potent than IL-17F at inducing downstream gene expression.32
IL-17F may also contribute to the psoriasis transcriptome. In an in vitro study in healthy skin explants treated with IL-17A, IL-17F, and IL-17A/F, the gene expression profile induced by IL-17F (and other IL-17 cytokines) significantly correlated with upregulation of the psoriasis transcriptome (MAD3-PSO; p<10-16).32 Therefore, IL-17F may also be considered a good therapeutic target, and bimekizumab, an antibody that neutralises both IL-17A and IL-17F and their heterodimers, is in clinical development for the treatment of psoriasis.33 Bimekizumab blocks IL-17 inflammatory pathways regulated by both IL-17A and IL-17F (Figure 2). Theoretically, however, there are still inflammatory signals regulated by IL-17C and IL-17E that could also contribute to psoriasis pathogenesis.
IL-17C
IL-17C is a proinflammatory cytokine that shares 23% sequence homology with IL-17A. It is produced by keratinocytes and is synergistically induced by IL-17A and TNF-α.34 IL-17C may synergise with other cytokines, such as TNF-α and IL-1β, and it binds to the IL-17C receptor, which consists of the IL-17RA and IL-17RE subunits (Figure 2).13,14 Expression of IL-17C mRNA in lesional skin is significantly higher than in unaffected and non-lesional skin.27 Interestingly, IL-17C protein levels are ~100-fold higher than IL-17A levels,27 suggesting that IL-17C is markedly active in stimulating inflammation in psoriatic skin.
The effects of IL-17C overlap with those of gene expression induction by IL-17A and IL-17F. IL-17C stimulates the genes in keratinocytes that have been previously described for IL-17A and IL-17F, namely cytokines, chemokines, and antimicrobial peptides.34 IL-17C may then generate an autoinflammatory loop because it is not only produced by, but can also act on, keratinocytes to produce downstream genes that are also regulated by IL-17A and IL-17F. Thus, IL-17C potentiates and amplifies IL-17A and IL-17F signals.20,34 IL-17C signalling contributes to psoriasis pathogenesis, albeit to a lesser extent than IL-17A and IL-17F. The gene expression profile induced by IL-17C in vitro weakly but significantly correlated with the upregulated psoriasis transcriptome (MAD3-PSO) in healthy skin explant (p<10-16).32
IL-17E
Lastly, IL-17E (also known as IL-25) is recognised as a therapeutic target in atopic dermatitis because it supported a Th2-mediated inflammatory response in a mouse model, stimulating expression of IL-4, IL-5, and IL-13.35 The pathogenic role of IL-17E in psoriasis is controversial, with contrasting data on its expression level in lesional psoriatic skin,27,36 as well as its effects on Th17 activation, as it is supposed to suppress IL-17A signalling.11 It binds to the IL-17E receptor, which consists of the IL-17RA and IL-17RB subunits (Figure 2).11,13
Previously, data did not support a role for an IL-17E-mediated pathway in psoriasis pathogenesis, as no upregulation of IL-17E was identified in lesional skin compared to non-lesional or normal skin,27 and no correlation was detected between the gene expression profile induced by IL-25E and the psoriasis transcriptome.32 Conversely, in another study, significantly higher IL-17E mRNA levels were found in lesional skin compared to non-lesional and unaffected skin, and keratinocytes were identified as a major source of IL-17E.36 Additionally, in vitro, IL-17E induced macrophages to express CCL20, IL-8, and TNF-α,36 which are genes central to psoriasis pathogenesis and inflammation. Hence, we may hypothesise an alternative inflammatory pathway in psoriasis that is driven by IL-17E and is not related to the main IL-17A pathway.
Blocking Inflammatory Pathways in Psoriasis
In psoriasis, multiple inflammatory pathways are driven by different IL-17 cytokines. The main pathway is driven by IL-17A and potentiated by IL-17F and IL-17C, plus a likely contribution from IL-17E. The only therapeutic strategy to simultaneously block all these inflammatory signals is blockade of the IL-17RA subunit, through which all of these cytokines signal (Figure 2).11,13,25,26 By blocking the IL-17RA subunit with an agent such as brodalumab, we can control all the inflammation regulated by IL-17 cytokines. The advantage of this approach compared with neutralising a single cytokine that only partially controls the IL-17 family activity needs to be confirmed, and mechanistic studies should be conducted to provide data to address this issue. Furthermore, head-to-head studies should be performed to determine whether there is any clinically meaningful difference in rapidity of effect, response duration, and safety in targeting IL-17RA over the cytokine.
What Difference Does a DLQI 0 or 1 Make to Patients? Are We Ambitious Enough?
Professor Matthias Augustin
Many patients with psoriasis do not receive optimal treatment, often waiting years to achieve relief of their symptoms. This was exemplified by a patient testimony video in which the patient described experiencing 10 years of uneven treatment before finally receiving biologic treatment and feeling well. This provided an example of the cumulative life course impairment patients experience. Thus, it is important to obtain the patient’s perspective and determine what difference achieving complete restitution of quality of life (i.e., a DLQI score of 0 or 1) would mean to them. As physicians, we should ask whether we are ambitious enough to help patients achieve this goal.
As advocated in the World Health Organization’s (WHO) Global Report on Psoriasis,37 dermatologists should have a patient-centred and holistic approach, beginning from their initial contact with patients. However, dermatologists may only have 10–20 minutes in their initial consultation with patients to identify their needs and to reach a treatment decision. Consequently, the availability of new psoriasis treatments is good for patients but challenging for dermatologists to make treatment choices in partnership with patients.
Why We Measure Patient-Reported Outcomes in Psoriasis
In evaluating treatment outcomes, we must not only consider objective outcomes but also the value to the patient.38 PRO provide a way of translating the outcomes of treatment decisions into value from the patient perspective and, therefore, provide support for the complex treatment decision-making process in psoriasis.
There are many tools to measure outcomes in psoriasis, such as objective, hybrid, and PRO,39 but we currently mainly use DLQI for quality of life assessment. Objective outcomes and PRO measures are both necessary because there is a degree of discrepancy between them. For example, in an early study evaluating the correlation of absolute PASI and DLQI scores in real-world care, no significant correlation was found between PASI and DLQI until the skin improved, and, at that point, DLQI also improved.40 DLQI was included in the 2011 European consensus of treatment goals for moderate-to-severe psoriasis.41 Although the treatment goal thresholds for PASI response are now higher, the principle remains of combining an objective measurement of treatment response with the patient perspective via DLQI to come to a treatment decision.41
The Use of DLQI
The DLQI consists of 10 questions and results in a score ranging from 0–30. The use of DLQI has been recommended in most guidelines,42 quality of care guidelines,43 and European registries.44 While complete clearance is the current goal, treatment goals should be agreed with the patient and should include quality of life measures. Indeed, data from 2,345 patients in the PsoBest German registry on the association between percentage improvement in PASI from baseline to 3 months and DLQI showed that greater proportions of patients with higher PASI response achieved DLQI 0 or 1, with almost 70% of patients who achieved PASI 100 reaching DLQI 0 or 1 (unpublished data).45
In routine practice, there are challenges associated with using DLQI, including determining the meaning of the DLQI score for the treatment decision. In fact, physicians should discuss the DLQI answers with the patient to focus on their most important needs, (e.g., reducing itch). A limitation of the DLQI is that 8 out of 10 questions allow a response of ‘not relevant’, which may lead to a bias in the sum score.
What Goals Should We Share with Our Patients?
When sharing treatment goals with patients, whether DLQI is enough to measure the patient perspective should be considered. To obtain a wider view of patient perspectives, 3,425 patients in large national healthcare studies in Germany were asked about their needs from treatment.46,47 The three most frequent answers were ‘to get better skin quickly’ (93%), ‘to be healed of all skin defects’ (91%), and ‘to have confidence in the therapy’ (89%), but patients listed many other items that they considered important.46,47 The Patient Benefit Index (PBI) was developed and has been used to evaluate the overall benefit as a sum of single benefits, such as ‘to be free of itch’.46,48
A further way to measure treatment benefit is to evaluate the association of PASI response, DLQI, and PBI with anchoring variables. Patients in the PsoBest registry were asked if they were ‘very satisfied with treatment’ after 3 months (unpublished data).45 Their response was used as the anchoring variable and a linear correlation was found between increasing PASI response, DLQI, and PBI benefit, and the proportion of patients who reported treatment satisfaction (unpublished data).45 Of note, the proportion of patients satisfied with treatment was much higher for those achieving DLQI 0 or 1 than DLQI 2–5. If ‘all skin lesions healed’ was used as the anchoring variable, it was achieved for ~80% of patients with DLQI 0 or 1 but <40% with DLQI 2–5 (unpublished data).
Some patients in PsoBest exhibited poor PASI response (PASI <50) but had a DLQI 0 or 1 (unpublished data).45 In this situation, potential bias in the DLQI sum score should be checked. The association of other PASI response measures (change in PASI score or absolute PASI score after 3 months) with DLQI was also evaluated, and absolute PASI score may best reflect the DLQI response.45 The achievement of DLQI0 or 1 was also associated with the greatest patient benefit, as assessed by PBI (unpublished data).45 In conclusion, DLQI 0 or 1 highly reflects patient benefit from treatment and the goal of reaching DLQI 0 or 1 should be integrated into clinical practice.







